Label: PENZAL Q- acetaminophen, ethenzamide, caffeine anhydrous tablet
-
Contains inactivated NDC Code(s)
NDC Code(s): 72689-0034-1 - Packager: OASIS TRADING
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated March 18, 2019
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
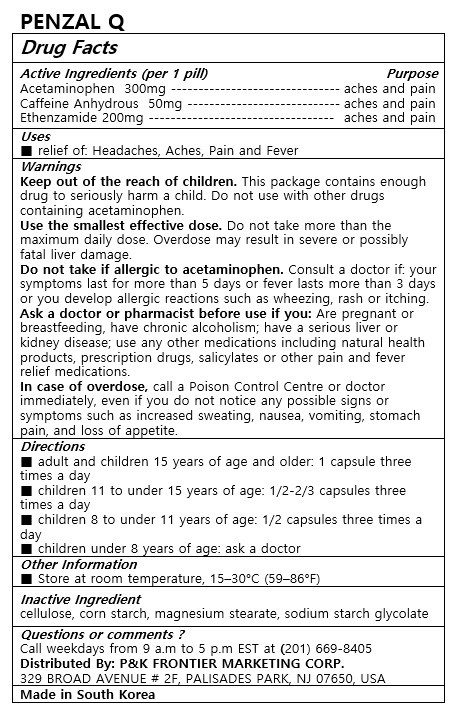
- ACTIVE INGREDIENT
- PURPOSE
- KEEP OUT OF REACH OF CHILDREN
- INDICATIONS & USAGE
-
WARNINGS
Keep out of the reach of children. This package contains enough drug to seriously harm a child. Do not use with other drugs containing acetaminophen.
Use the smallest effective dose. Do not take more than the maximum daily dose. Overdose may result in severe or possibly fatal liver damage.
Do not take if allergic to acetaminophen. Consult a doctor if: your symptoms last for more than 5 days or fever lasts more than 3 days or you develop allergic reactions such as wheezing, rash or itching.
Ask a doctor or pharmacist before use if you: Are pregnant or breastfeeding, have chronic alcoholism; have a serious liver or kidney disease; use any other medications including natural health products, prescription drugs, salicylates or other pain and fever relief medications.
In case of overdose, call a Poison Control Centre or doctor immediately, even if you do not notice any possible signs or symptoms such as increased sweating, nausea, vomiting, stomach pain, and loss of appetite.
WARNING: Do not use if carton is open or if printed bottle neck band or inner foil seal is broken.
Store at room temperature, 15–30°C (59–86°F). - INACTIVE INGREDIENT
- DOSAGE & ADMINISTRATION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
PENZAL Q
acetaminophen, ethenzamide, caffeine anhydrous tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:72689-0034 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ETHENZAMIDE (UNII: L929ZCK4BF) (ETHENZAMIDE - UNII:L929ZCK4BF) ETHENZAMIDE 200 mg ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 300 mg CAFFEINE (UNII: 3G6A5W338E) (CAFFEINE - UNII:3G6A5W338E) CAFFEINE 50 mg Inactive Ingredients Ingredient Name Strength SODIUM STARCH GLYCOLATE TYPE A CORN (UNII: AG9B65PV6B) POWDERED CELLULOSE (UNII: SMD1X3XO9M) MAGNESIUM STEARATE (UNII: 70097M6I30) STARCH, CORN (UNII: O8232NY3SJ) Product Characteristics Color yellow Score 2 pieces Shape OVAL Size 17mm Flavor Imprint Code PenzalQ Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72689-0034-1 10 in 1 BLISTER PACK; Type 0: Not a Combination Product 11/20/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part343 11/20/2018 Labeler - OASIS TRADING (689991468) Registrant - OASIS TRADING (689991468) Establishment Name Address ID/FEI Business Operations OASIS TRADING 689991468 manufacture(72689-0034)