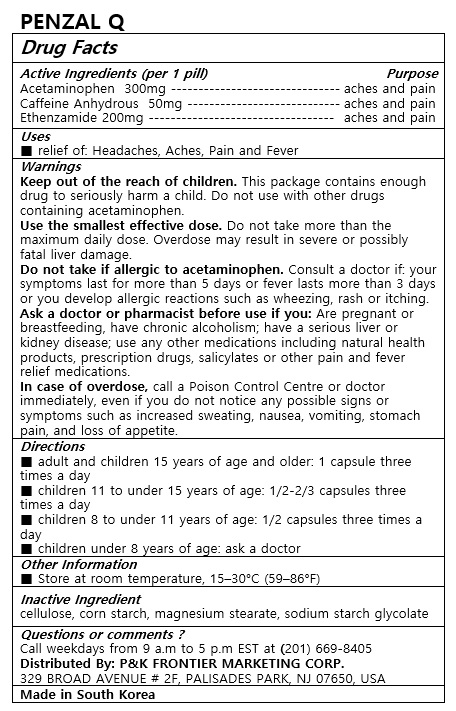
■ adult and children 15 years of age and older: 1 capsules three times a day
■ children 11 to under 15 years of age: 1/2-2/3 capsules three times a day
■ children 8 to under 11 years of age: 1/2 capsules three times a day
■ children under 8 years of age: ask a doctor
Keep out of the reach of children. This package contains enough drug to seriously harm a child. Do not use with other drugs containing acetaminophen.
Use the smallest effective dose. Do not take more than the maximum daily dose. Overdose may result in severe or possibly fatal liver damage.
Do not take if allergic to acetaminophen. Consult a doctor if: your symptoms last for more than 5 days or fever lasts more than 3 days or you develop allergic reactions such as wheezing, rash or itching.
Ask a doctor or pharmacist before use if you: Are pregnant or breastfeeding, have chronic alcoholism; have a serious liver or kidney disease; use any other medications including natural health products, prescription drugs, salicylates or other pain and fever relief medications.
In case of overdose, call a Poison Control Centre or doctor immediately, even if you do not notice any possible signs or symptoms such as increased sweating, nausea, vomiting, stomach pain, and loss of appetite.
WARNING: Do not use if carton is open or if printed bottle neck band or inner foil seal is broken.
Store at room temperature, 15–30°C (59–86°F).