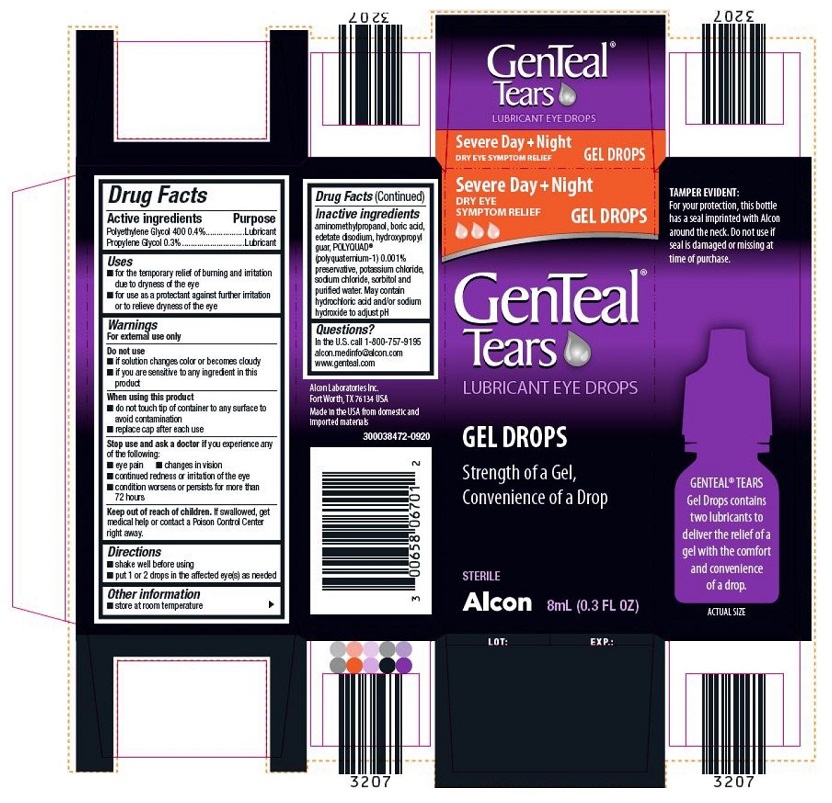
Label: GENTEAL TEARS GEL DROPS- polyethylene glycol 400 and propylene glycol gel
- NDC Code(s): 0065-8067-01
- Packager: Alcon Laboratories, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 14, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- Uses
- Warnings
- Do not use
- When using this product
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
- Directions
- Other information
- Inactive ingredients
- Questions?
-
PRINCIPAL DISPLAY PANEL
Severe Day + Night
DRY EYE SYMPTOM RELIEF GEL DROPS
GenTeaL® Tears
LUBRICANT EYE DROPS
GEL DROPS
Strength of a Gel,
Convenience of a drop
STERILE
Alcon 8ml (0.3 FL OZ)
TAMPER EVIDENT:
For your protection, this bottle has a seal imprinted with Alcon around the neck. Do not use if seal is damaged or missing at time of purchase.
GENTEAL® TEARS Gel Drops contains two lubricants to deliver the relief of a gel with the comfort and convenience of a drop.
-
INGREDIENTS AND APPEARANCE
GENTEAL TEARS GEL DROPS
polyethylene glycol 400 and propylene glycol gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0065-8067 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Polyethylene Glycol 400 (UNII: B697894SGQ) (Polyethylene Glycol 400 - UNII:B697894SGQ) Polyethylene Glycol 400 4 mg in 1 mL Propylene Glycol (UNII: 6DC9Q167V3) (Propylene Glycol - UNII:6DC9Q167V3) Propylene Glycol 3 mg in 1 mL Inactive Ingredients Ingredient Name Strength Aminomethylpropanol (UNII: LU49E6626Q) Boric Acid (UNII: R57ZHV85D4) Edetate Disodium (UNII: 7FLD91C86K) Guaraprolose (3500 Mpa.S At 1%) (UNII: 3A1I7376TC) Polidronium Chloride (UNII: 6716Z5YR3G) Potassium Chloride (UNII: 660YQ98I10) Sodium Chloride (UNII: 451W47IQ8X) Sorbitol (UNII: 506T60A25R) Water (UNII: 059QF0KO0R) Hydrochloric Acid (UNII: QTT17582CB) Sodium Hydroxide (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0065-8067-01 8 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product 02/04/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M018 02/04/2021 Labeler - Alcon Laboratories, Inc. (008018525) Establishment Name Address ID/FEI Business Operations Alcon Research LLC 007672236 manufacture(0065-8067)