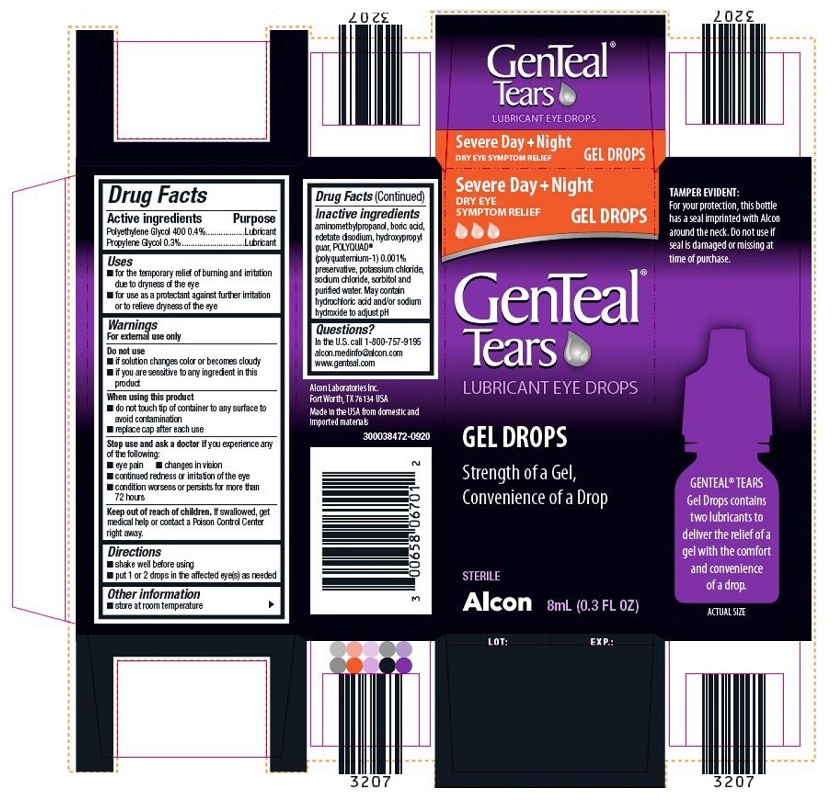
Uses
- for the temporary relief of burning and irritation due to dryness of the eye
- for use as a protectant against further irritation or to relieve dryness of the eye
Do not use
- if this product changes color or becomes cloudy
- if you are sensitive to any ingredient in this product
When using this product
- do not touch tip of container to any surface to avoid contamination
- replace cap after each use
Stop use and ask a doctor if you experience any of the following:
- eye pain
- changes in vision
- continued redness or irritation of the eye
- condition worsens or persists for more than 72 hours
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Inactive ingredients
Aminomethylpropanol, boric acid, edetate disodium, hydroxypropyl guar, POLYQUAD® (polyquaternium-1) 0.001% preservative, potassium chloride, sodium chloride, sorbitol and purified water. May contain hydrochloric acid and /or sodium hydroxide to adjust pH.
Questions?
In the U.S. call
1-800-757-9195
alcon.medinfo@alcon.com
www.genteal.com
Alcon Laboratories Inc.
Fort Worth, TX 76134 USA
Made in the USA from domestic and imported materials
300038472-0920
TOP OF THE CARTON
GenTeal® Tears
LUBRICANT EYE DROPS
Severe Day + Night
DRY EYE SYMPTOM RELIEF GEL DROPS
PRINCIPAL DISPLAY PANEL
Severe Day + Night
DRY EYE SYMPTOM RELIEF GEL DROPS
GenTeaL® Tears
LUBRICANT EYE DROPS
GEL DROPS
Strength of a Gel,
Convenience of a drop
STERILE
Alcon 8ml (0.3 FL OZ)
TAMPER EVIDENT:
For your protection, this bottle has a seal imprinted with Alcon around the neck. Do not use if seal is damaged or missing at time of purchase.
GENTEAL® TEARS Gel Drops contains two lubricants to deliver the relief of a gel with the comfort and convenience of a drop.