Label: BENZETHONIUM CHLORIDE AND DYCLONINE HYDROCHLORIDE- liquid bandage liquid
- NDC Code(s): 69842-084-30
- Packager: CVS Pharmacy
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated January 24, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
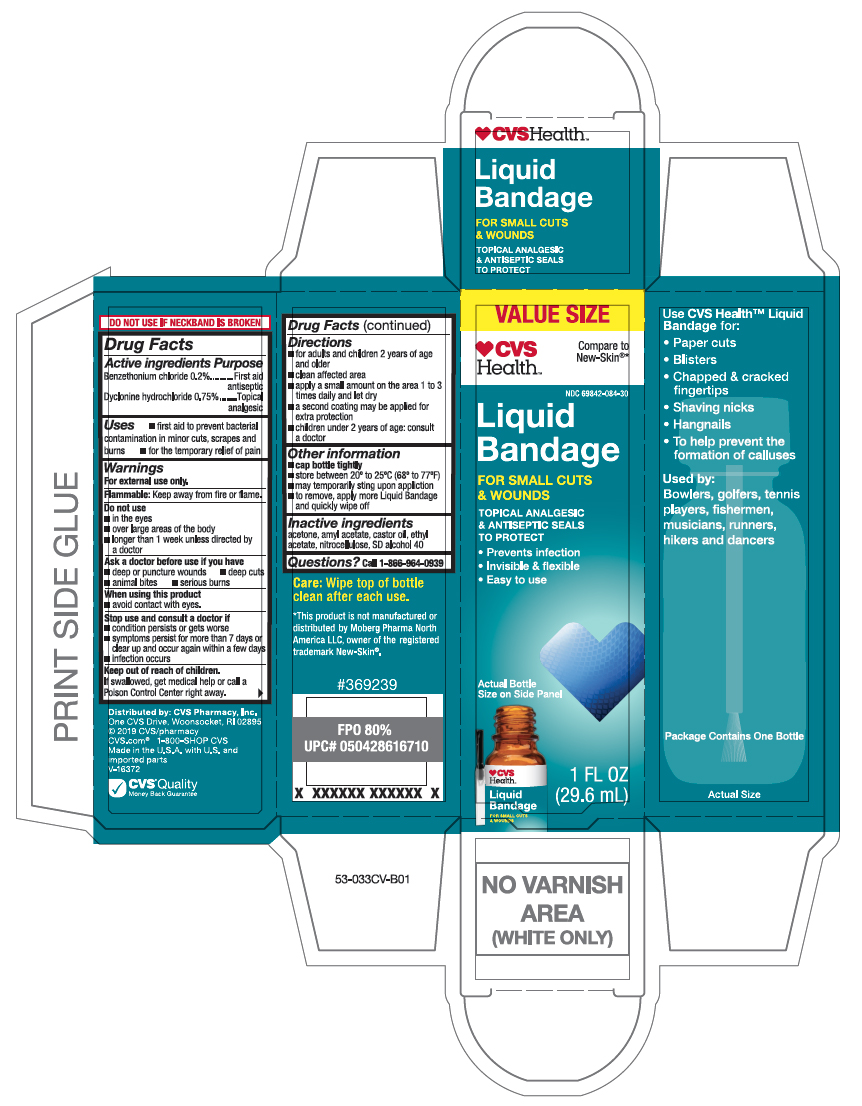
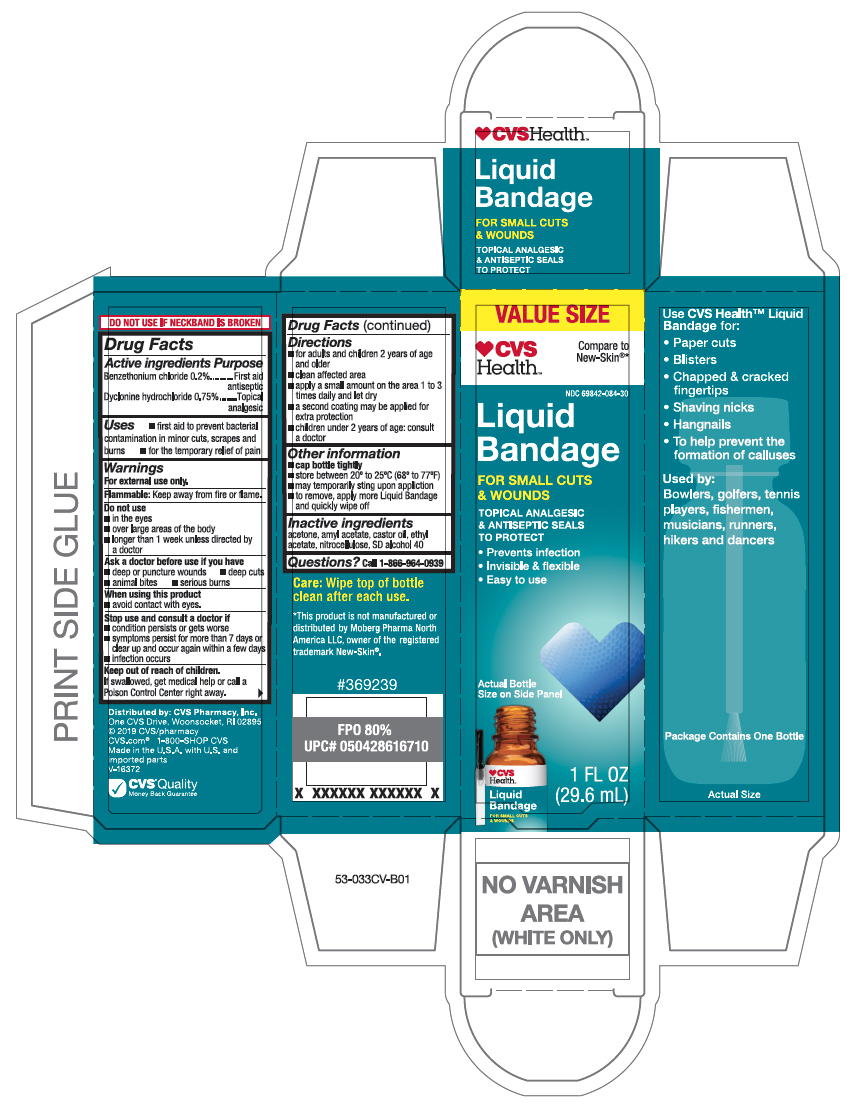
- Active ingredients
- Purpose
- Uses
- Warnings
- Directions
- Other information
- Inactive ingredient
- Questions?
- Principal Display Panel
-
INGREDIENTS AND APPEARANCE
BENZETHONIUM CHLORIDE AND DYCLONINE HYDROCHLORIDE
liquid bandage liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69842-084 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZETHONIUM CHLORIDE (UNII: PH41D05744) (BENZETHONIUM - UNII:1VU15B70BP) BENZETHONIUM CHLORIDE 0.2 mg in 10 mL DYCLONINE HYDROCHLORIDE (UNII: ZEC193879Q) (DYCLONINE - UNII:078A24Q30O) DYCLONINE HYDROCHLORIDE 0.75 mg in 10 mL Inactive Ingredients Ingredient Name Strength PYROXYLIN (UNII: KYR8BR2X6O) ALCOHOL (UNII: 3K9958V90M) CASTOR OIL (UNII: D5340Y2I9G) ACETONE (UNII: 1364PS73AF) AMYL ACETATE (UNII: 92Q24NH7AS) ETHYL ACETATE (UNII: 76845O8NMZ) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69842-084-30 10 mL in 1 BOTTLE, WITH APPLICATOR; Type 0: Not a Combination Product 06/01/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M003 06/01/2019 Labeler - CVS Pharmacy (062312574)