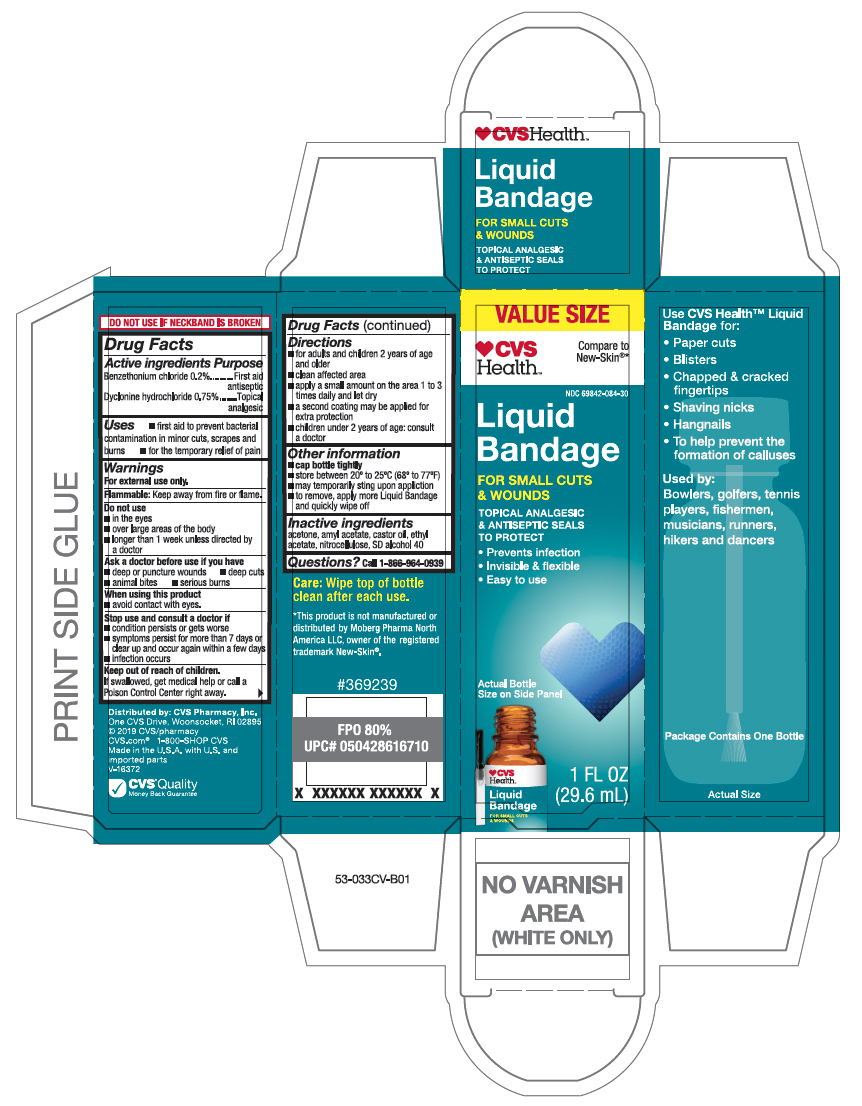
Active ingredients
Benzethonium chloride 0.2%
Dyclonine hydrochloride 0.75%
Purpose
First aid antiseptic
Topical analgesic
Uses
- first aid to prevent bacterial contamination in minor cuts, scrapes and burns
- for the temporary relief of pain
Warnings
For external use only.
Flammable:
Keep away from fire or flame.
Do not use
- in the eyes
- over large areas of the body
- longer than 1 week unless directed by a doctor
Ask a doctor before use if you have
- deep or puncture wounds
- deep cuts
- animal bites
- serious burns
Stop use and consult a doctor if
- condition persists or gets worse
- symptoms persist for more than 7 days or clear up an occur again within a few days
- infection occurs
Keep out of reach of children.
If swallowed, get medical help or call a Poison Control Center right away.
Directions
- for adults and children 2 years of age and older
- clean affected area
- apply a small amount on the area 1 to 3 times daily and let dry
- a second coating may be applied for extra protection
- children under 2 years of age: consult a doctor
Other information
- cap tightly
- store between 20º to 25º C (68º to 77ºF)
- may temporarily sting upon application
- to remove, apply more Liquid Bandage and quickly wipe off
Inactive ingredient
acetone, amyl acetate, castor oil, ethyl acetate, nitrocellulose, SD alcohol 40
Questions?
Call 1-866-964-0939
Principal Display Panel
CVS Health
Liquid Bandage
FOR SMALL CUTS & WOUNDS
TOPICAL ANALGESIC & ANTISEPTIC SEALS TO PROTECT
- Prevent infection
- Invisible & flexible
- Easy to use
Actual Bottle Size on Side Panel
1 FL OZ (29.6 mL)