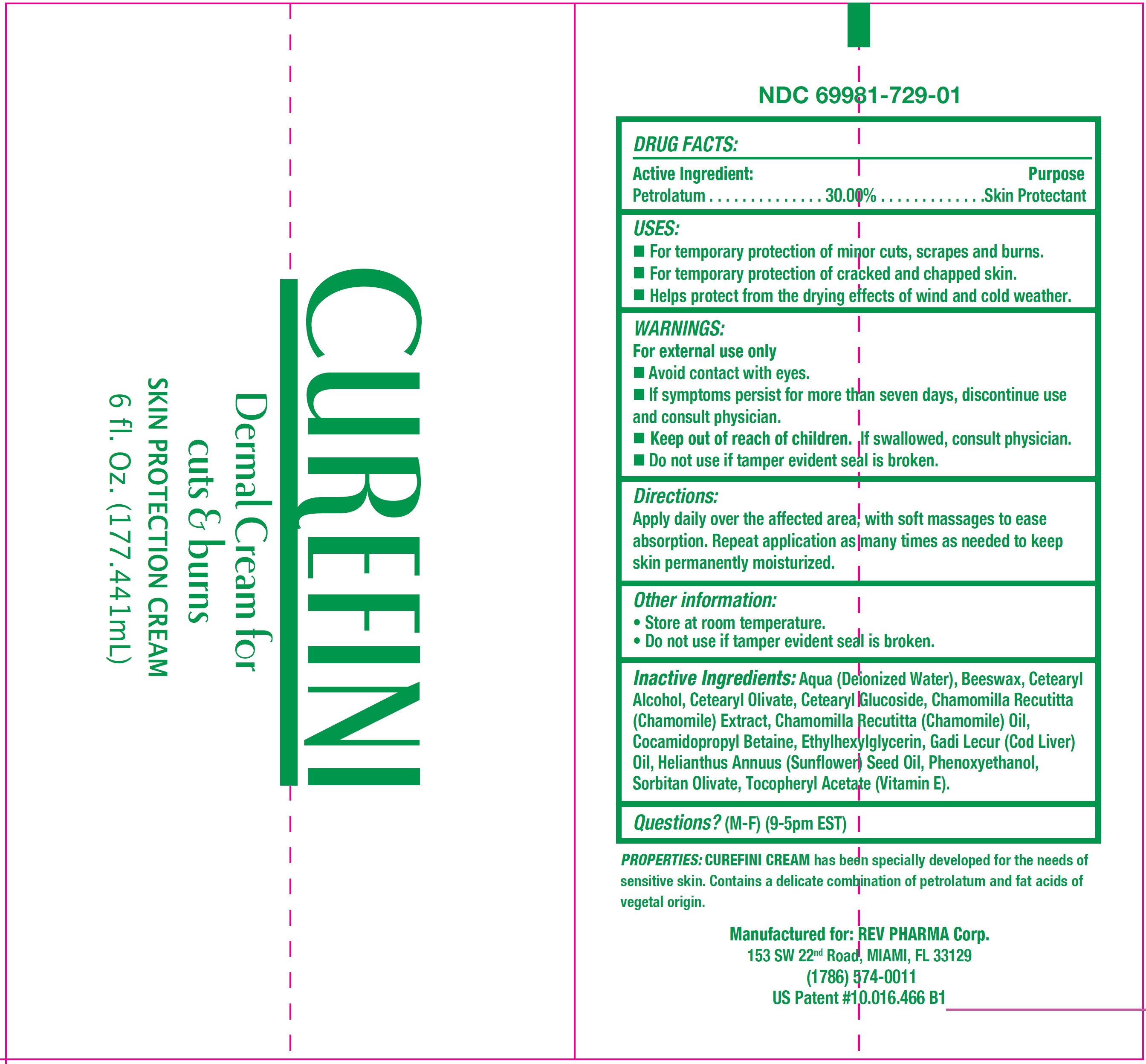
Label: CUREFINI DERMAL- petrolatum cream
- NDC Code(s): 69981-729-01, 69981-729-03
- Packager: Rev Pharma Corp.
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated November 10, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- DRUG FACTS:
- Active Ingredient:
- USES:
- WARNINGS:
- Directions:
- Other information:
-
Inactive Ingredients:
Aqua (Deionized Water), Beeswax, Cetearyl Alcohol, Cetearyl Olivate, Cetearyl Glucoside, Chamomilla Recutitta (Chamomile) Extract, Chamomilla Recutitta (Chamomile) Oil, Cocamidopropyl Betaine, Ethylhexylglycerin, Gadi Lecur (Cod Liver) Oil, Helianthus Annuus ( Sunflower) Seed Oil, Phenoxyethanol, Sorbitan Olivate, Tocopheryl Acetate (Vitamin E).
- Questions?
- Package Labeling: 177.441mL (69981-729-01)
- Package Labeling:90ml (69981-729-03)
-
INGREDIENTS AND APPEARANCE
CUREFINI DERMAL
petrolatum creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69981-729 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PETROLATUM (UNII: 4T6H12BN9U) (PETROLATUM - UNII:4T6H12BN9U) PETROLATUM 300 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) YELLOW WAX (UNII: 2ZA36H0S2V) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) CETEARYL OLIVATE (UNII: 58B69Q84JO) CETEARYL GLUCOSIDE (UNII: 09FUA47KNA) CHAMOMILE (UNII: FGL3685T2X) CHAMOMILE FLOWER OIL (UNII: 60F80Z61A9) COCAMIDOPROPYL BETAINE (UNII: 5OCF3O11KX) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) COD LIVER OIL (UNII: BBL281NWFG) SUNFLOWER OIL (UNII: 3W1JG795YI) PHENOXYETHANOL (UNII: HIE492ZZ3T) SORBITAN OLIVATE (UNII: MDL271E3GR) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69981-729-01 1 in 1 BOX 02/08/2017 1 177.441 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 2 NDC:69981-729-03 90 mL in 1 TUBE; Type 0: Not a Combination Product 11/01/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M016 02/08/2017 Labeler - Rev Pharma Corp. (079422405)