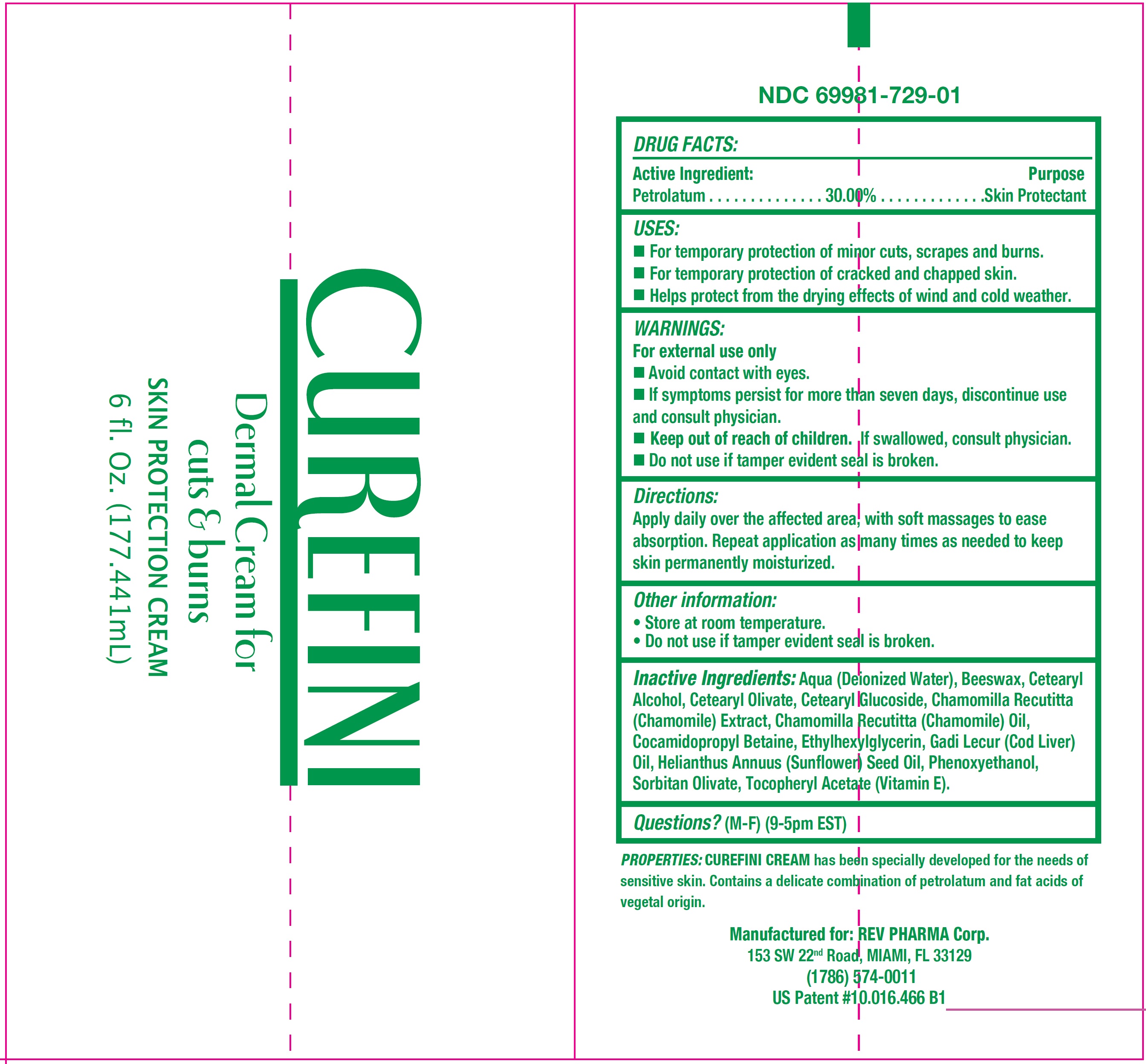
USES:
- For temporary protection of minor cuts, scrapes and burns.
- For temporary protection of cracked and chapped skin.
- Helps protect from drying caused by wind and cold weather.
WARNINGS:
For external use only
- Avoid contact with eyes.
- If symptoms persist for more than seven days, discontinue use and consult physician.
Directions:
Apply daily over the affected area, with soft massages to ease absorption. Repeat application as many times as needed to keep skin permanently moisturized.
Inactive Ingredients:
Aqua (Deionized Water), Beeswax, Cetearyl Alcohol, Cetearyl Olivate, Cetearyl Glucoside, Chamomilla Recutitta (Chamomile) Extract, Chamomilla Recutitta (Chamomile) Oil, Cocamidopropyl Betaine, Ethylhexylglycerin, Gadi Lecur (Cod Liver) Oil, Helianthus Annuus ( Sunflower) Seed Oil, Phenoxyethanol, Sorbitan Olivate, Tocopheryl Acetate (Vitamin E).