Label: TRICLARA- benzalkonium chloride liquid
- NDC Code(s): 72518-001-00
- Packager: Nightingale Pharmaceuticals, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 28, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
-
DOSAGE & ADMINISTRATION
Directions
- clean the affected area with soap and water and thoroughly dry the area.
- for best results apply a small quantity with a rubbing motion 8 times (once every 2 hours while awake) daily at onset of symptoms until cleared.
- wash hands after applying.
- do not share this product with others.
- retain these directions for help with use.
- for use on children under 12 years: ask a doctor.
- OTHER SAFETY INFORMATION
- INACTIVE INGREDIENT
-
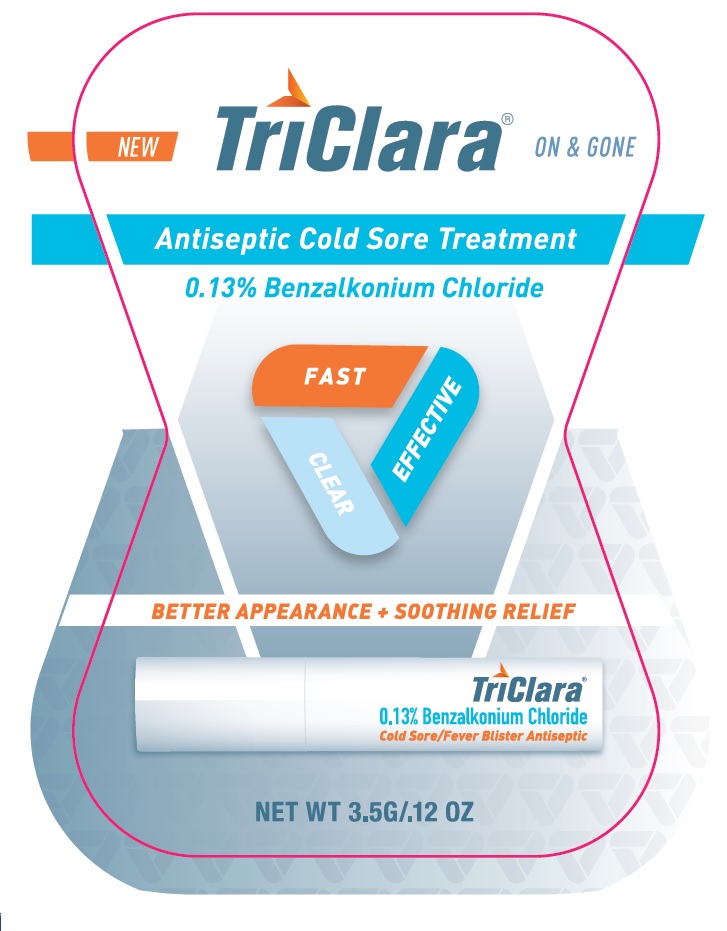
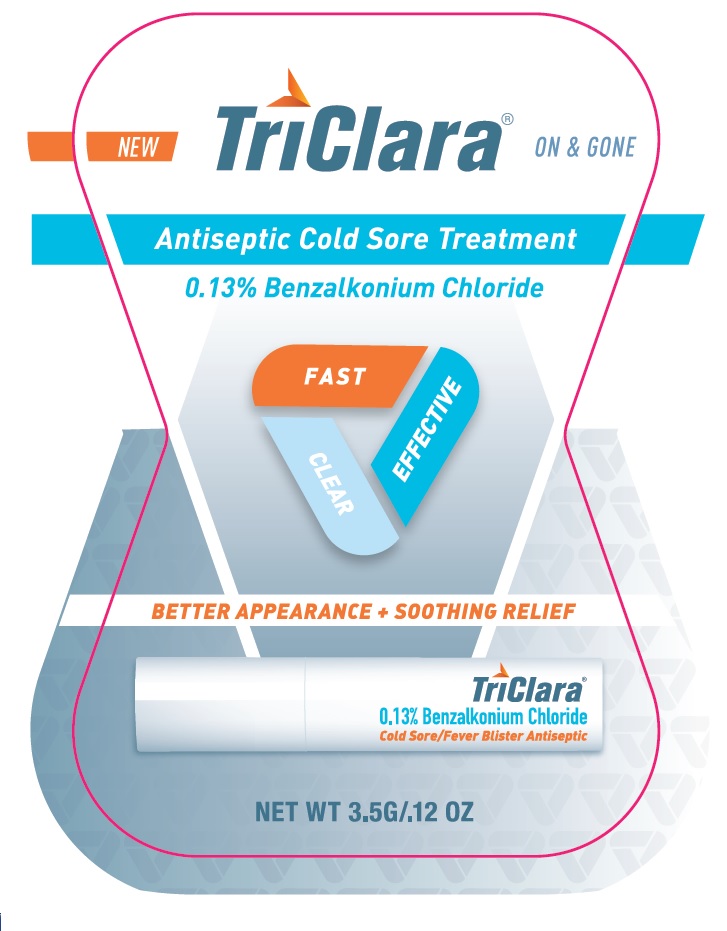
PRINCIPAL DISPLAY PANEL
TriClara ® ON & GONE
New
Antiseptic Cold Sore Treatment
0.13% Benzalkonium Chloride
FAST
CLEAR
EFFECTIVE
BETTER APPEARANCE + SOOTHING RELIEF
Cold Sore / Fever Blister Antiseptic
NET WT 3.5G / .12 OZ
Distributed by:
Nightingale Pharmaceuticals, Inc.
Santee, CA 92071
www.triclara.com
RETAIN CARTON FOR COMPLETE PRODUCT INFORMAITON

-
INGREDIENTS AND APPEARANCE
TRICLARA
benzalkonium chloride liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:72518-001 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 0.13 g in 100 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ACETYLCYSTEINE (UNII: WYQ7N0BPYC) GLYCERIN (UNII: PDC6A3C0OX) SODIUM HYDROXIDE (UNII: 55X04QC32I) ASCORBIC ACID (UNII: PQ6CK8PD0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72518-001-00 3.5 g in 1 BOTTLE; Type 0: Not a Combination Product 05/14/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 05/14/2019 Labeler - Nightingale Pharmaceuticals, Inc. (081338827)