Uses
- treatment of cold sore / fever blister
- effective relief of symptoms of cold sore / fever blister
- protects against infections in sores, burns, cuts and scrapes
Warnings
- For external use only: Do not use in or around eyes or ears. Consult a physician before using on puncture wounds, serious burns or animal bites.
- Allergy alert: Do not use if you are allergic to any ingredients in this product.
Keep out of reach of children. If swallowed, get medical help or contact poison control at 1-800-222-1222.
Directions
- clean the affected area with soap and water and thoroughly dry the area.
- for best results apply a small quantity with a rubbing motion 8 times (once every 2 hours while awake) daily at onset of symptoms until cleared.
- wash hands after applying.
- do not share this product with others.
- retain these directions for help with use.
- for use on children under 12 years: ask a doctor.
Other information
- Store at 15 to 25°C (59 to 77°F), in a dry place, away from heat, humidity and direct sunlight
- Discard 30 days after removeal from vauumed pouch.
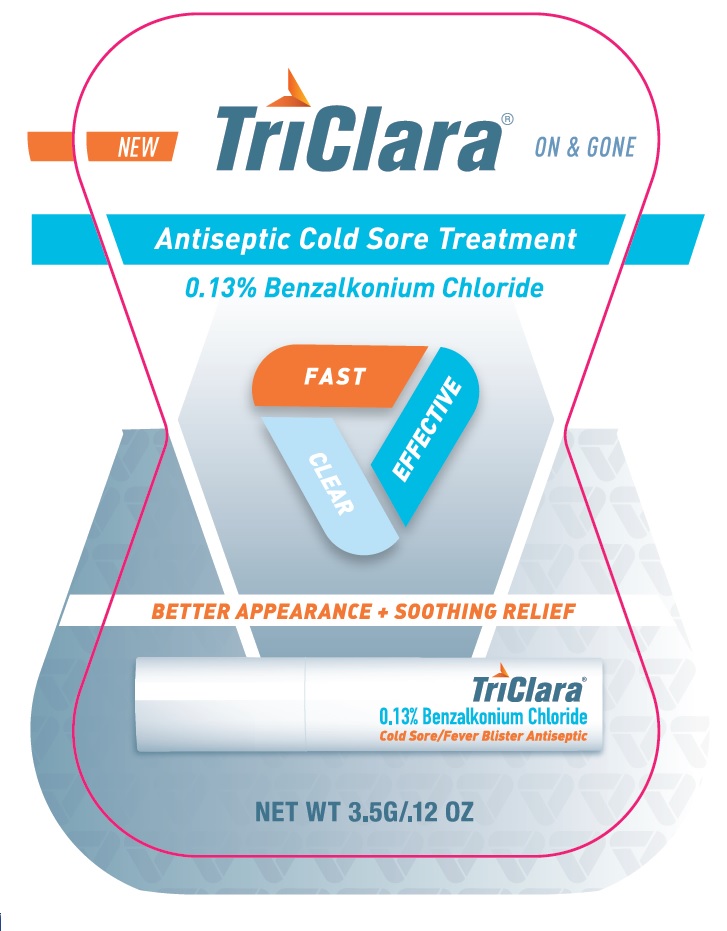
TriClara ® ON & GONE
New
Antiseptic Cold Sore Treatment
0.13% Benzalkonium Chloride
FAST
CLEAR
EFFECTIVE
BETTER APPEARANCE + SOOTHING RELIEF
Cold Sore / Fever Blister Antiseptic
NET WT 3.5G / .12 OZ
Distributed by:
Nightingale Pharmaceuticals, Inc.
Santee, CA 92071
www.triclara.com
RETAIN CARTON FOR COMPLETE PRODUCT INFORMAITON