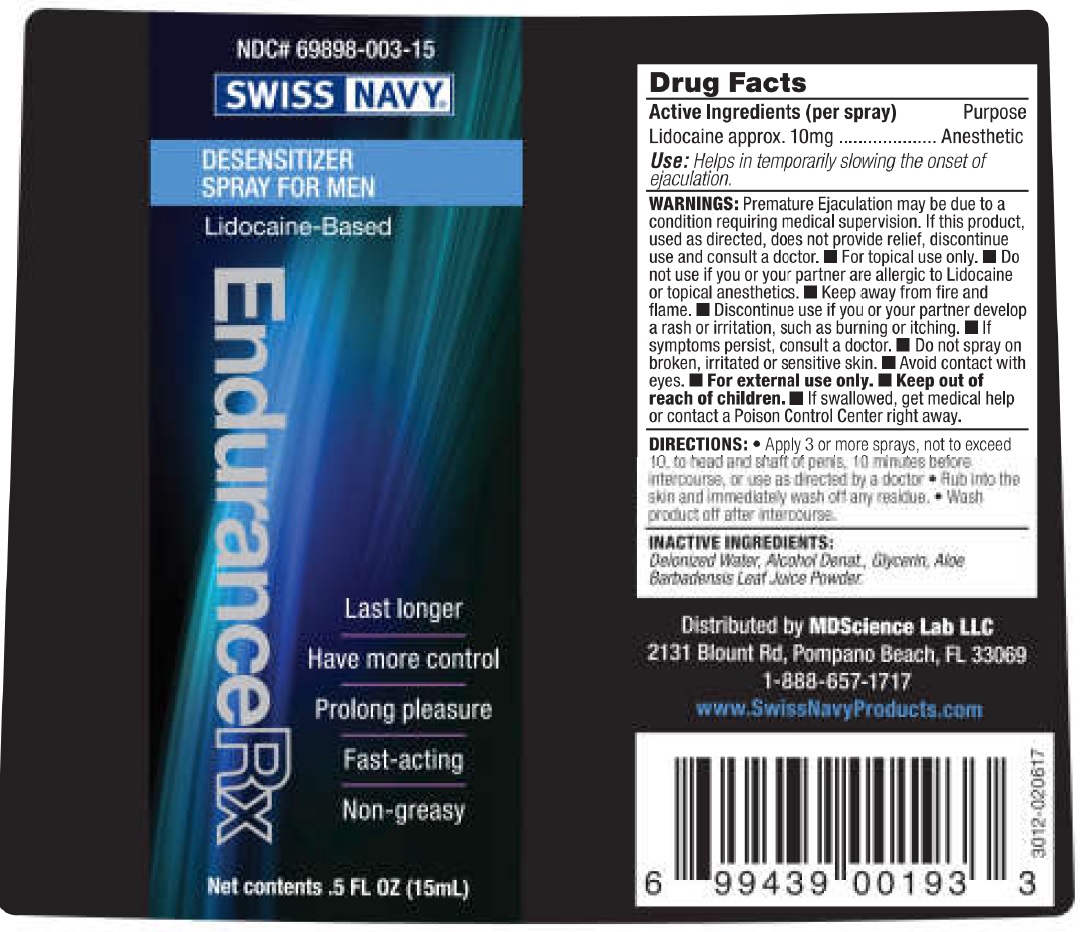
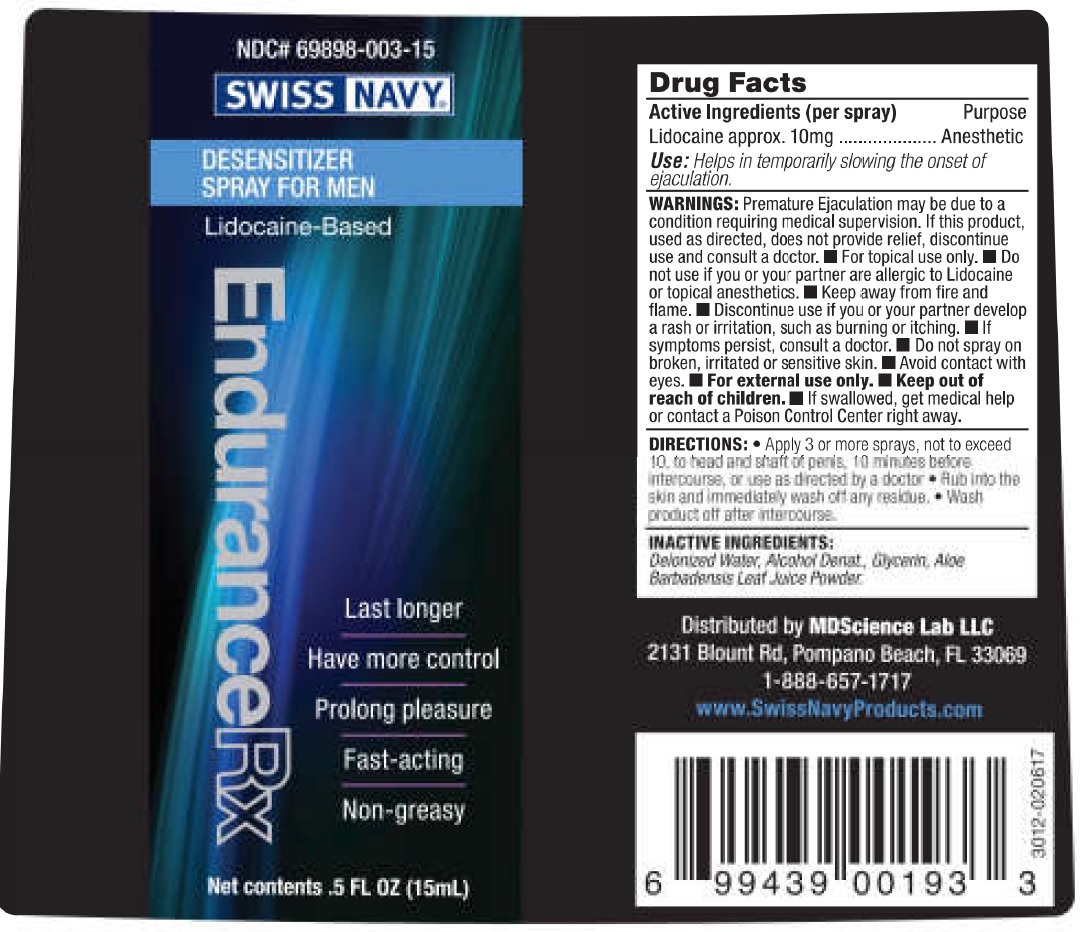
Label: SWISS NAVY ENDURANCE RX- lidocaine spray
- NDC Code(s): 69872-003-15
- Packager: PACKAGE SOLUTIONS LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 19, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active Ingredients (per spray)
- Use:
-
WARNINGS:
Premature Ejaculation may be due to a condition requiring medical supervision. If this product, used as directed, does not provide relief, discontinue use and consult a doctor.
- For topical use only.
Do not use
- if you or your partner are allergic to Lidocaine or topical anesthetics.
- Keep away from fire and flame.
- Discontinue use if you or your partner develop a rash or irritation, such as burning or itching.
- If symptoms persist, consult a doctor.
- Do not spray on broken, irritated or sensitive skin.
- Avoid contact with eyes.
- For external use only.
- DIRECTIONS:
- INACTIVE INGREDIENTS:
- Packgae Labeling:
-
INGREDIENTS AND APPEARANCE
SWISS NAVY ENDURANCE RX
lidocaine sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69872-003 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LIDOCAINE (UNII: 98PI200987) (LIDOCAINE - UNII:98PI200987) LIDOCAINE 10 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ALCOHOL (UNII: 3K9958V90M) GLYCERIN (UNII: PDC6A3C0OX) ALOE VERA LEAF (UNII: ZY81Z83H0X) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69872-003-15 1 in 1 BOX 08/01/2017 1 15 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 08/01/2017 Labeler - PACKAGE SOLUTIONS LLC (054705875)