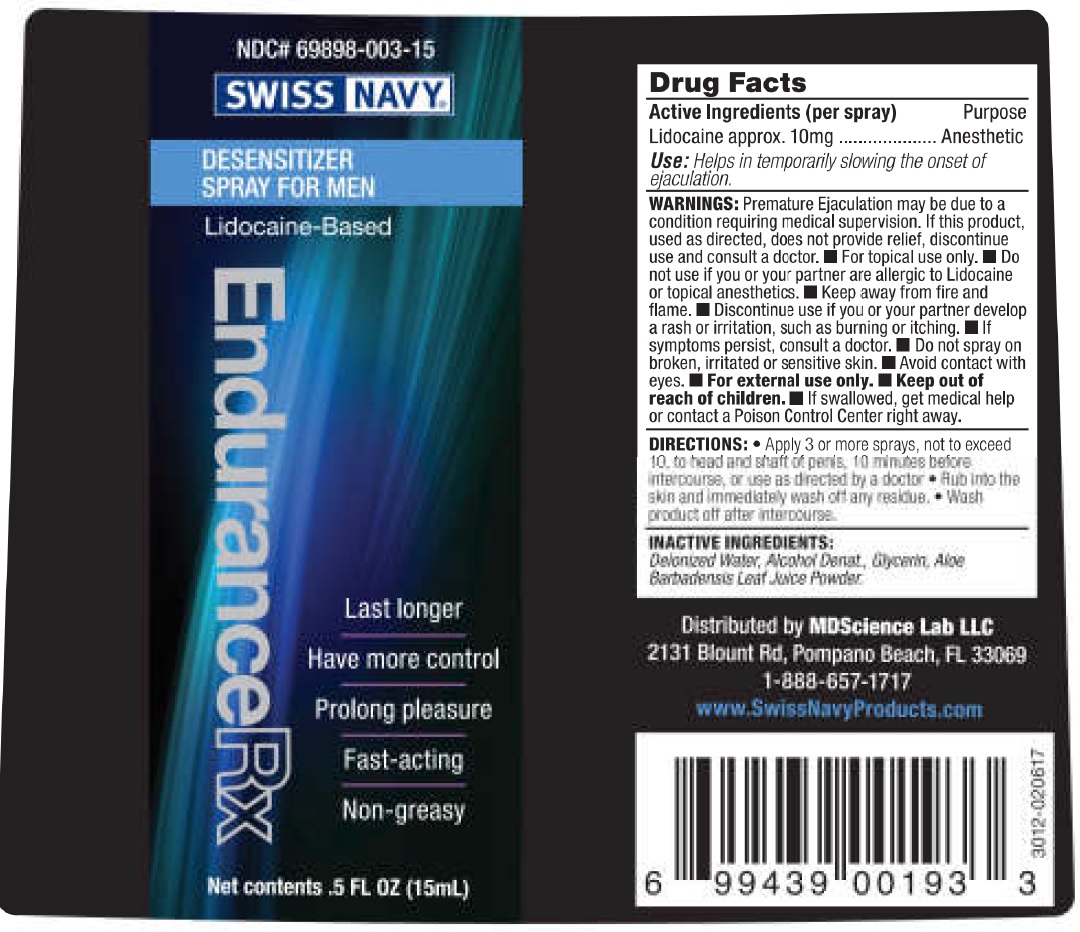
WARNINGS:
Premature Ejaculation may be due to a condition requiring medical supervision. If this product, used as directed, does not provide relief, discontinue use and consult a doctor.
- For topical use only.
Do not use
- if you or your partner are allergic to Lidocaine or topical anesthetics.
- Keep away from fire and flame.
- Discontinue use if you or your partner develop a rash or irritation, such as burning or itching.
- If symptoms persist, consult a doctor.
- Do not spray on broken, irritated or sensitive skin.
- Avoid contact with eyes.
- For external use only.
DIRECTIONS:
Apply 3 or more sprays, not to exceed to 10, to head and shaft of penis, 10 minutes before intercourse, or use as directed by a doctor
Rub into the skin and immediately wash off any residue.
Wash product off after intercourse.