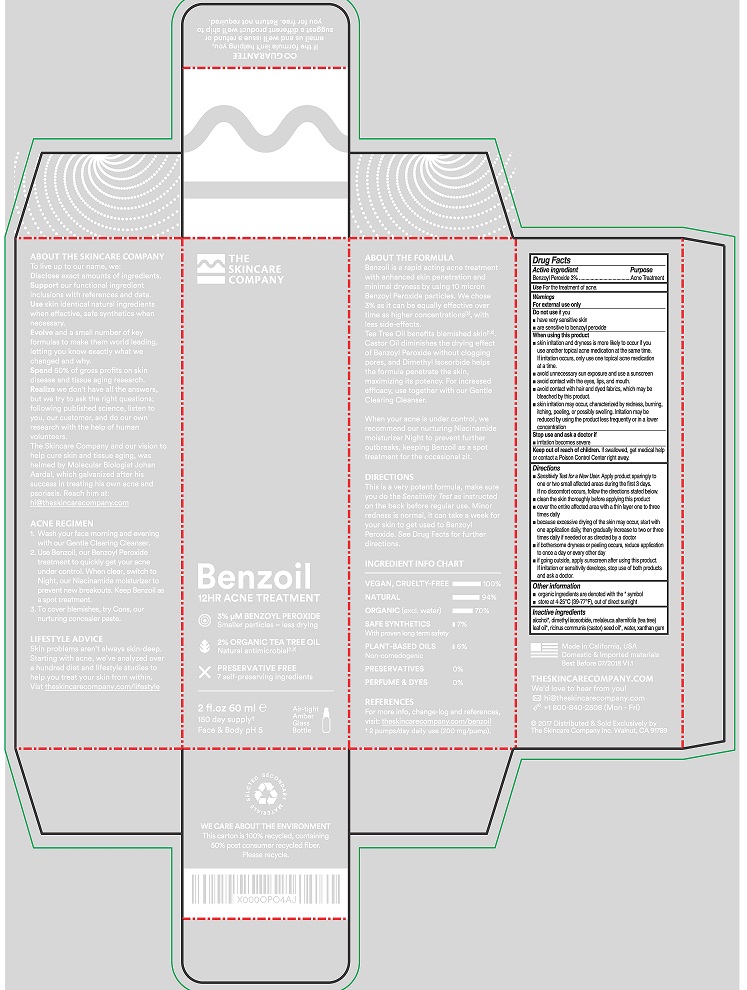
Label: BENZOIL 12HR ACNE TREATMENT- benzoyl peroxide lotion
- NDC Code(s): 71792-101-11
- Packager: THE SKINCARE COMPANY, INC.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated January 7, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- USE
- WARNINGS
-
WHEN USING
WHEN USING THIS PRODUCT
- SKIN IRRITATION AND DRYNESS IS MORE LIKELY TO OCCUR IF YOU USE ANOTHER TOPICAL ACNE MEDICATION AT THE SAME TIME. IF IRRITATION OCCURS, ONLY USE ONE TOPICAL ACNE MEDICATION AT A TIME.
- AVOID UNNECESSARY SUN EXPOSURE AND USE A SUNSCREEN.
- AVOID CONTACT WITH THE EYES, LIPS, AND MOUTH.
- AVOID CONTACT WITH HAIR AND DYED FABRICS, WHICH MAY BE BLEACHED BY THIS PRODUCT.
- SKIN IRRITATION MAY OCCUR, CHARACTERIZED BY REDNESS, BURNING, ITCHING, PEELING, OR POSSIBLY SWELLING. IRRITATION MAY BE REDUCED BY USING THE PRODUCT LESS FREQUENTLY OR IN A LOWER CONCENTRATION.
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
-
DIRECTIONS
- SENSITIVITY TEST FOR A NEW USER. APPLY PRODUCT SPARINGLY TO ONE OR TWO SMALL AFFECTED AREAS DURING THE FIRST 3 DAYS. IF NO DISCOMFORT OCCURS, FOLLOW THE DIRECTIONS STATED BELOW.
- CLEAN THE SKIN THOROUGHLY BEFORE APPLYING THIS PRODUCT.
- COVER THE ENTIRE AFFECTED AREA WITH A THIN LAYER ONE TO THREE TIMES DAILY.
- BECAUSE EXCESSIVE DRYING OF THE SKIN MAY OCCUR, START WITH ONE APPLICATION DAILY, THEN GRADUALLY INCREASE TO TWO OR THREE TIMES DAILY IF NEEDED OR AS DIRECTED BY A DOCTOR.
- IF BOTHERSOME DRYNESS OR PEELING OCCURS, REDUCE APPLICATION TO ONCE A DAY OR EVERY OTHER DAY.
- IF GOING OUTSIDE, APPLY SUNSCREEN AFTER USING THIS PRODUCT. IF IRRITATION OR SENSITIVITY DEVELOPS, STOP USE OF BOTH PRODUCTS AND ASK A DOCTOR.
- OTHER INFORMATION
- INACTIVE INGREDIENTS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
BENZOIL 12HR ACNE TREATMENT
benzoyl peroxide lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:71792-101 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZOYL PEROXIDE (UNII: W9WZN9A0GM) (BENZOYL PEROXIDE - UNII:W9WZN9A0GM) BENZOYL PEROXIDE 3 g in 100 mL Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) DIMETHYL ISOSORBIDE (UNII: SA6A6V432S) MELALEUCA ALTERNIFOLIA LEAF (UNII: G43C57162K) CASTOR OIL (UNII: D5340Y2I9G) WATER (UNII: 059QF0KO0R) XANTHAN GUM (UNII: TTV12P4NEE) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:71792-101-11 60 mL in 1 TUBE; Type 0: Not a Combination Product 10/12/2017 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M006 10/12/2017 Labeler - THE SKINCARE COMPANY, INC. (314521346)