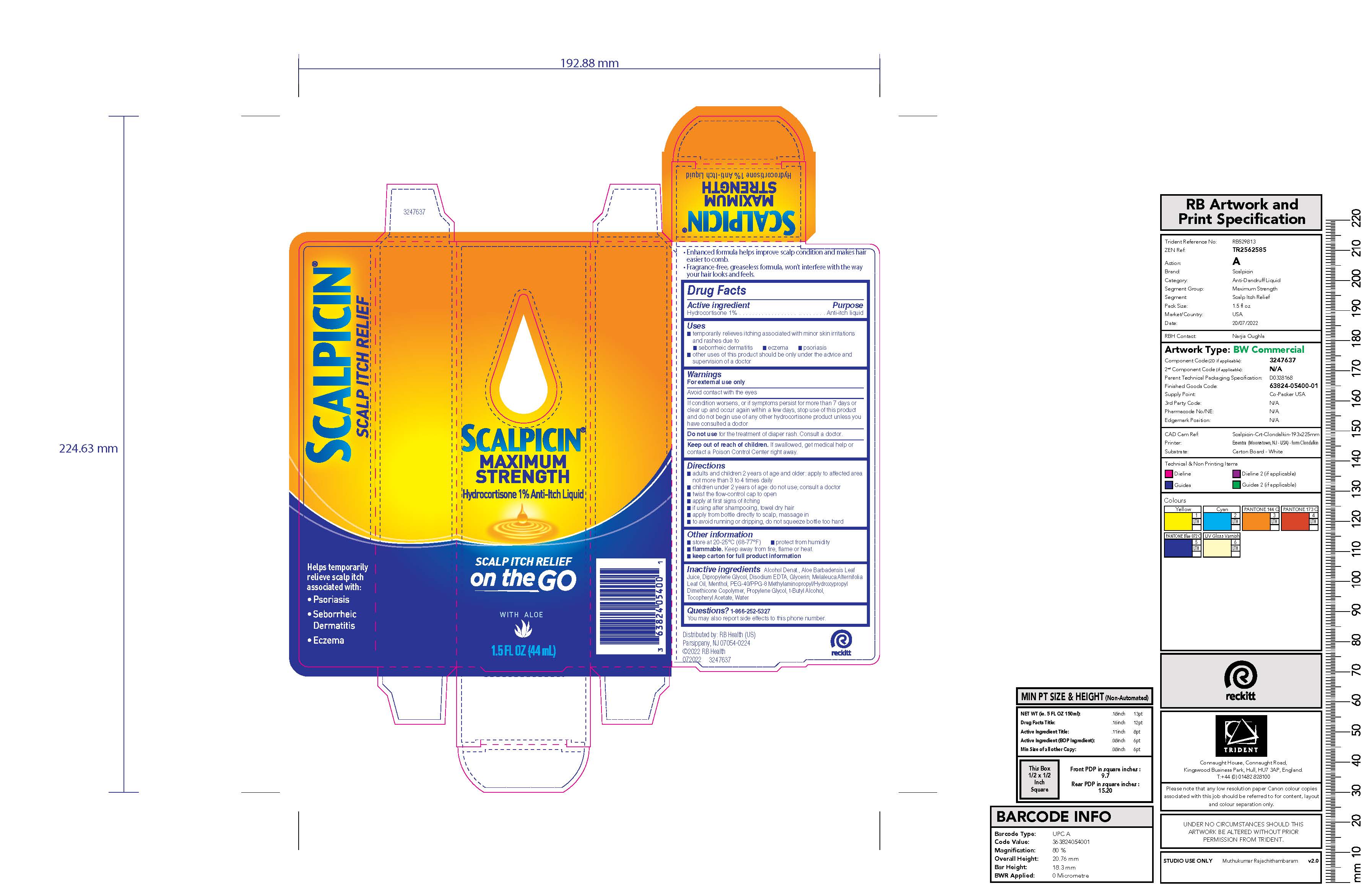
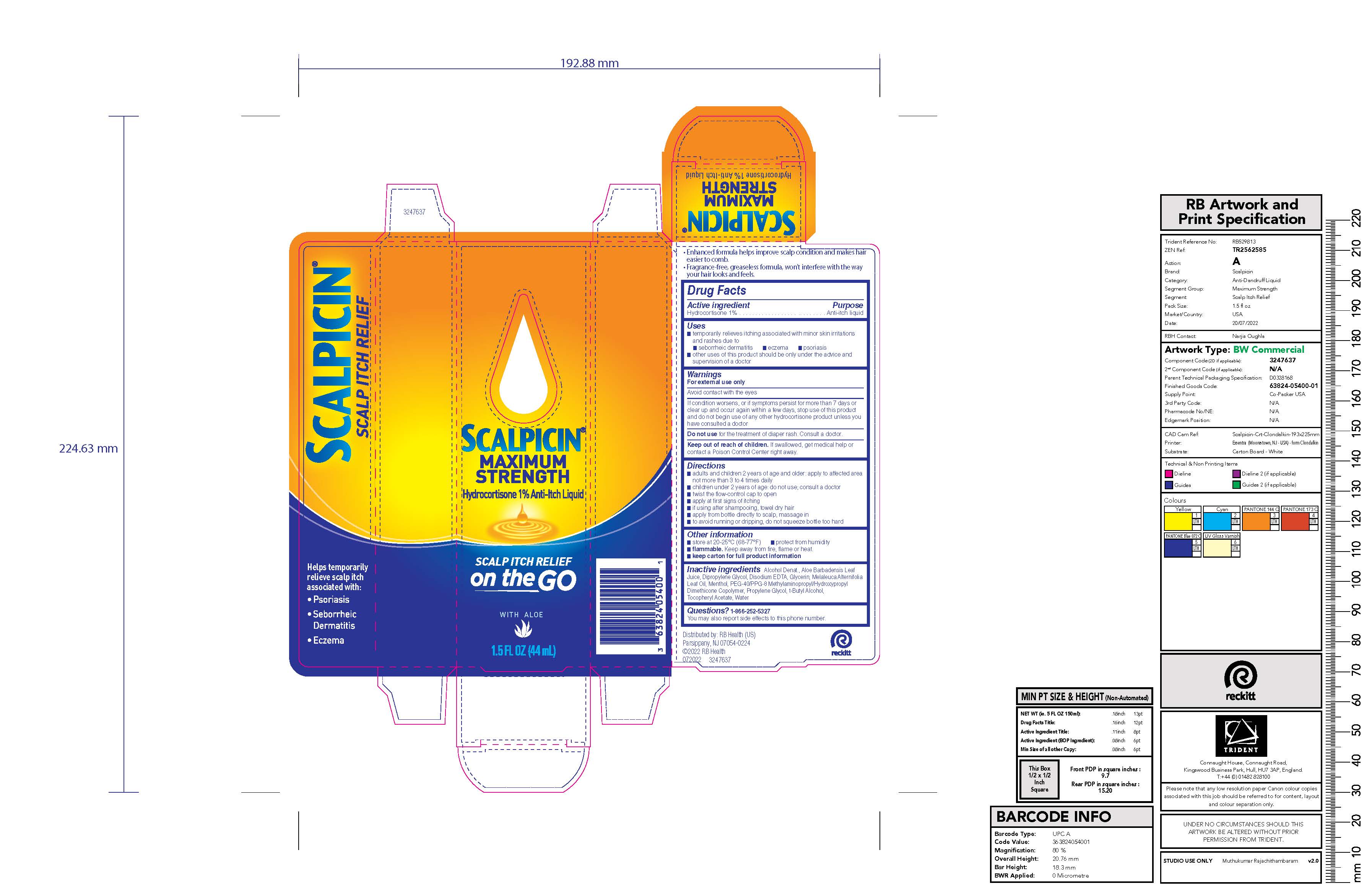
Label: SCALPICIN ANTI-ITCH MAXIMUM STRENGTH- hydrocortisone liquid
- NDC Code(s): 63824-851-15
- Packager: RB Health (US) LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated January 12, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purpose
- Uses
- Warnings
-
Directions
- adults and children 2 years of age and older: apply to affected area not more than 3 to 4 times daily
- children under 2 years of age: do not use; consult a doctor
- twist the flow-control cap to open
- apply at first signs of itching
- if using after shampooing, towel dry hair
- apply from bottle directly to scalp, massage in
- to avoid running or dripping, do not squeeze bottle too hard
- Other information
- Inactive ingredients
- Questions?
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 44 mL Bottle Carton
-
INGREDIENTS AND APPEARANCE
SCALPICIN ANTI-ITCH MAXIMUM STRENGTH
hydrocortisone liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:63824-851 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HYDROCORTISONE (UNII: WI4X0X7BPJ) (HYDROCORTISONE - UNII:WI4X0X7BPJ) HYDROCORTISONE 1 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) GLYCERIN (UNII: PDC6A3C0OX) DIPROPYLENE GLYCOL (UNII: E107L85C40) MENTHOL, UNSPECIFIED FORM (UNII: L7T10EIP3A) EDETATE DISODIUM (UNII: 7FLD91C86K) ALOE VERA LEAF (UNII: ZY81Z83H0X) ISOPROPYL ALCOHOL (UNII: ND2M416302) TEA TREE OIL (UNII: VIF565UC2G) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:63824-851-15 1 in 1 CARTON 10/15/2012 1 44 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 10/15/2012 Labeler - RB Health (US) LLC (081049410)