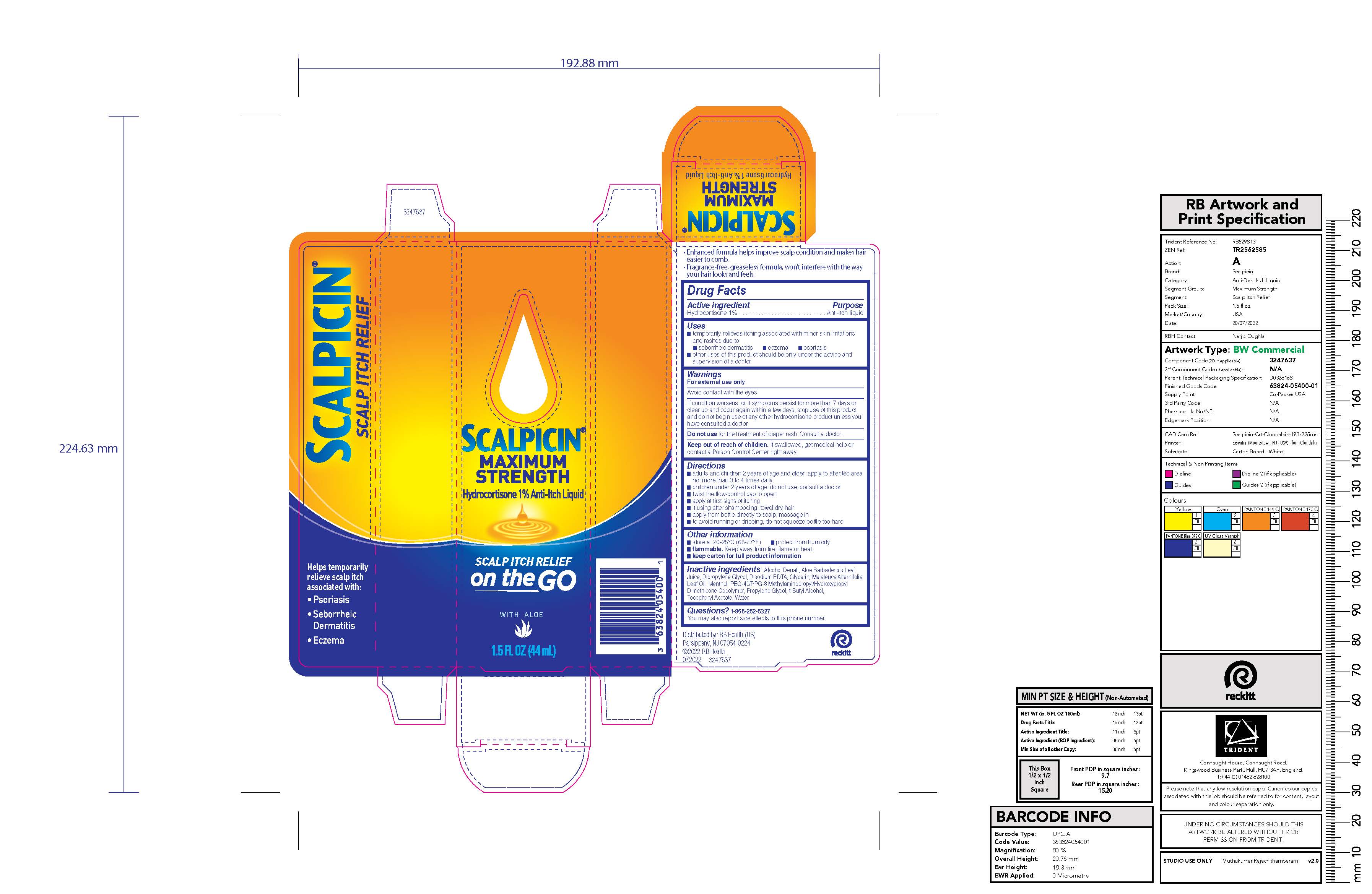
SCALPICIN ANTI-ITCH MAXIMUM STRENGTH- hydrocortisone liquid
RB Health (US) LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active ingredient
Hydrocortisone 1%
Uses
- temporarily relieves itching associated with minor skin irritations and rashes due to
- seborrheic dermatitis
- eczema
- psoriasis
- other uses of this product should be only under the advice and supervision of a doctor
Warnings
For external use only
Avoid contact with the eyes
If condition worsens, or if symptoms persist for more than 7 days or clear up and occur again within a few days, stop use of this product and do not begin use of any other hydrocortisone product unless you have consulted a doctor
Do not use for the treatment of diaper rash. Consult a doctor.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- adults and children 2 years of age and older: apply to affected area not more than 3 to 4 times daily
- children under 2 years of age: do not use; consult a doctor
- twist the flow-control cap to open
- apply at first signs of itching
- if using after shampooing, towel dry hair
- apply from bottle directly to scalp, massage in
- to avoid running or dripping, do not squeeze bottle too hard
Other information
- store at 20-25 °C (68-77°F)
- protect from humidity
-
flammable. Keep away from fire, flame or heat.
-
keep carton for full product information
Inactive ingredients
Alcohol Denat., Aloe Barbadensis Leaf Juice, Dipropylene Glycol, Disodium EDTA, Glycerin, Melaleuca Alternifolia Leaf Oil, Menthol, PEG-40/PPG-8 Methylaminopropyl/Hydroxypropyl Dimethicone Copolymer, Propylene Glycol, t-Butyl Alcohol, Tocopheryl Acetate, Water
Questions?
1-866-252-5327
You may also report side effects to this phone number.
Distributed by: RB Health (US)
Parsippany, NJ 07054-0224
PRINCIPAL DISPLAY PANEL - 44 mL Bottle Carton
SCALPICIN
®
MAXIMUM
STRENGTH
Hydrocortisone 1% Anti-Itch Liquid
SCALP ITCH RELIEF
on the GO
WITH ALOE
1.5 FL OZ (44 mL)