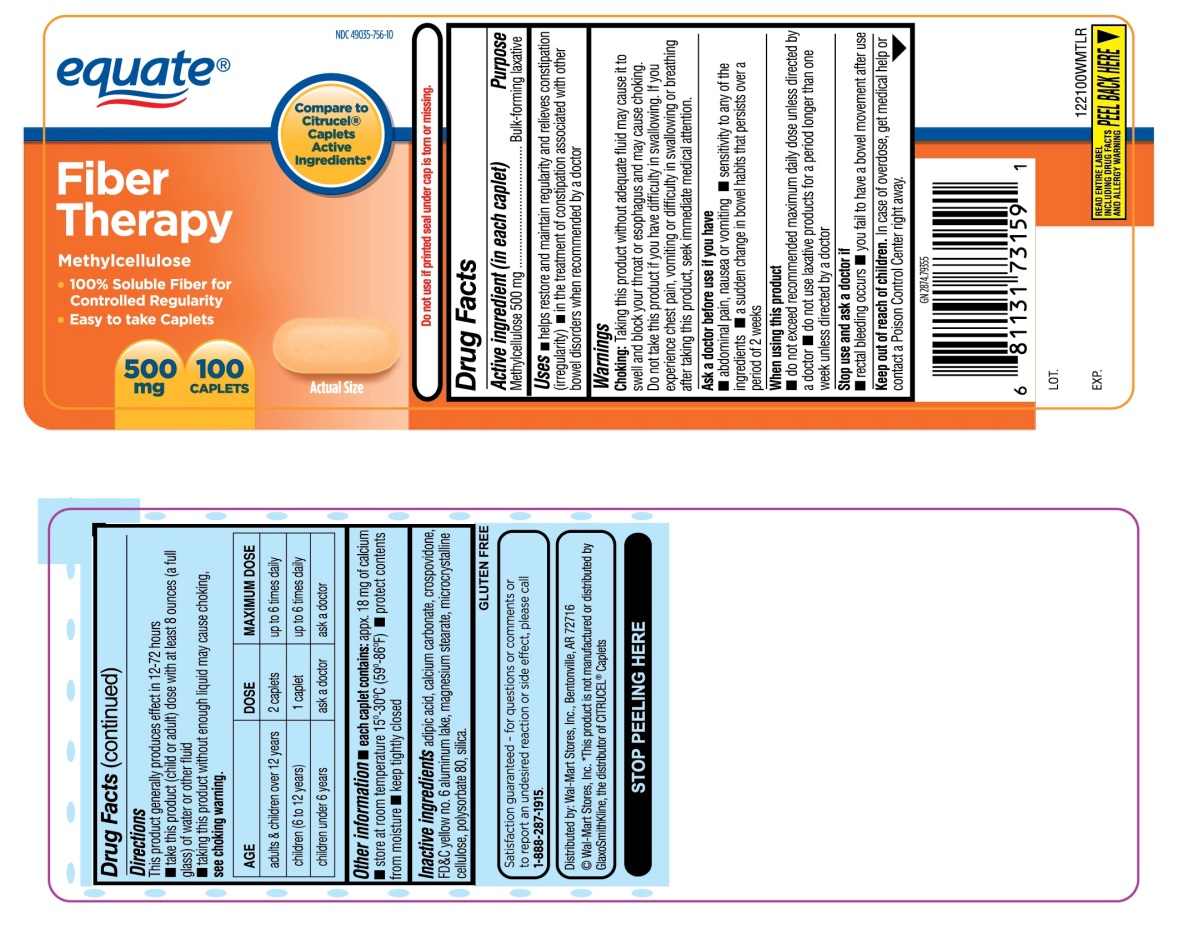
Label: EQUATE FIBER THERAPY- methylcellulose tablet
- NDC Code(s): 49035-756-10
- Packager: Wal-Mart Stores,Inc.,
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 12, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient (in each caplet)
- Purpose
-
Uses
- ▪
- helps restore and maintain regularity and relieves constipation (irregularity)
- ▪
- in the treatment of constipation associated with other bowel disorders when recommended by a doctor
Warnings
Choking: Taking this product without adequate fluid may cause it to swell and block your throat or esophagus and may cause choking. Do not take this product if you have difficulty in swallowing. If you experience chest pain, vomiting or difficulty in swallowing or breathing after taking this product, seek immediate medical attention.
Ask a doctor before use if you have
- ▪
- abdominal pain, nausea or vomiting
- ▪
- sensitivity to any of the ingredients
- ▪
- a sudden change in bowel habits that persists over a period of 2 weeks
-
Directions
This product generally produces effect in 12-72 hours
- ▪
- take this product (child or adult) dose with at least 8 ounces (a full glass) of water or other fluid
- ▪
- taking this product without enough liquid may cause choking.
- See choking warning.
AGE
DOSE
MAXIMUM DOSE
adults & children over 12 years
2 caplets
up to 6 times daily
children( 6 to 12 years )
1 caplet.
up to 6 times daily
children under 6 years
ask a doctor
ask a doctor
- Other information
- Inactive ingredients
-
Principal Display Panel
NDC 49035-756-10
Compare to Citrucel® Caplets Active Ingredients*
equate®
Fiber Therapy
Methylcellulose
- •
- 100% Soluble Fiber for Controlled Regularity
- •
- Easy to take Caplets
- 500 mg 100 CAPLETS
GLUTEN FREE
Satisfaction guaranteed-for questions or comments or to report an undesired reaction or side effect, please call 1-888-287-1915.
Distributed by:
Wal-Mart Stores, Inc., Bentonville, AR 72716
©Wal-Mart Stores, Inc.
*This product is not manufactured or distributed by GlaxoSmithKline, the distributor of Citrucel® Caplets.
-
INGREDIENTS AND APPEARANCE
EQUATE FIBER THERAPY
methylcellulose tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:49035-756 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength METHYLCELLULOSE (4000 MPA.S) (UNII: MRJ667KA5E) (METHYLCELLULOSE (4000 MPA.S) - UNII:MRJ667KA5E) METHYLCELLULOSE (4000 MPA.S) 500 mg Inactive Ingredients Ingredient Name Strength ADIPIC ACID (UNII: 76A0JE0FKJ) CALCIUM CARBONATE (UNII: H0G9379FGK) CROSPOVIDONE (120 .MU.M) (UNII: 68401960MK) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) POLYSORBATE 80 (UNII: 6OZP39ZG8H) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) Product Characteristics Color ORANGE (Light orange) Score no score Shape CAPSULE (Caplet) Size 19mm Flavor Imprint Code RP122 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:49035-756-10 100 in 1 BOTTLE; Type 0: Not a Combination Product 08/30/2017 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M007 08/30/2017 Labeler - Wal-Mart Stores,Inc., (051957769)