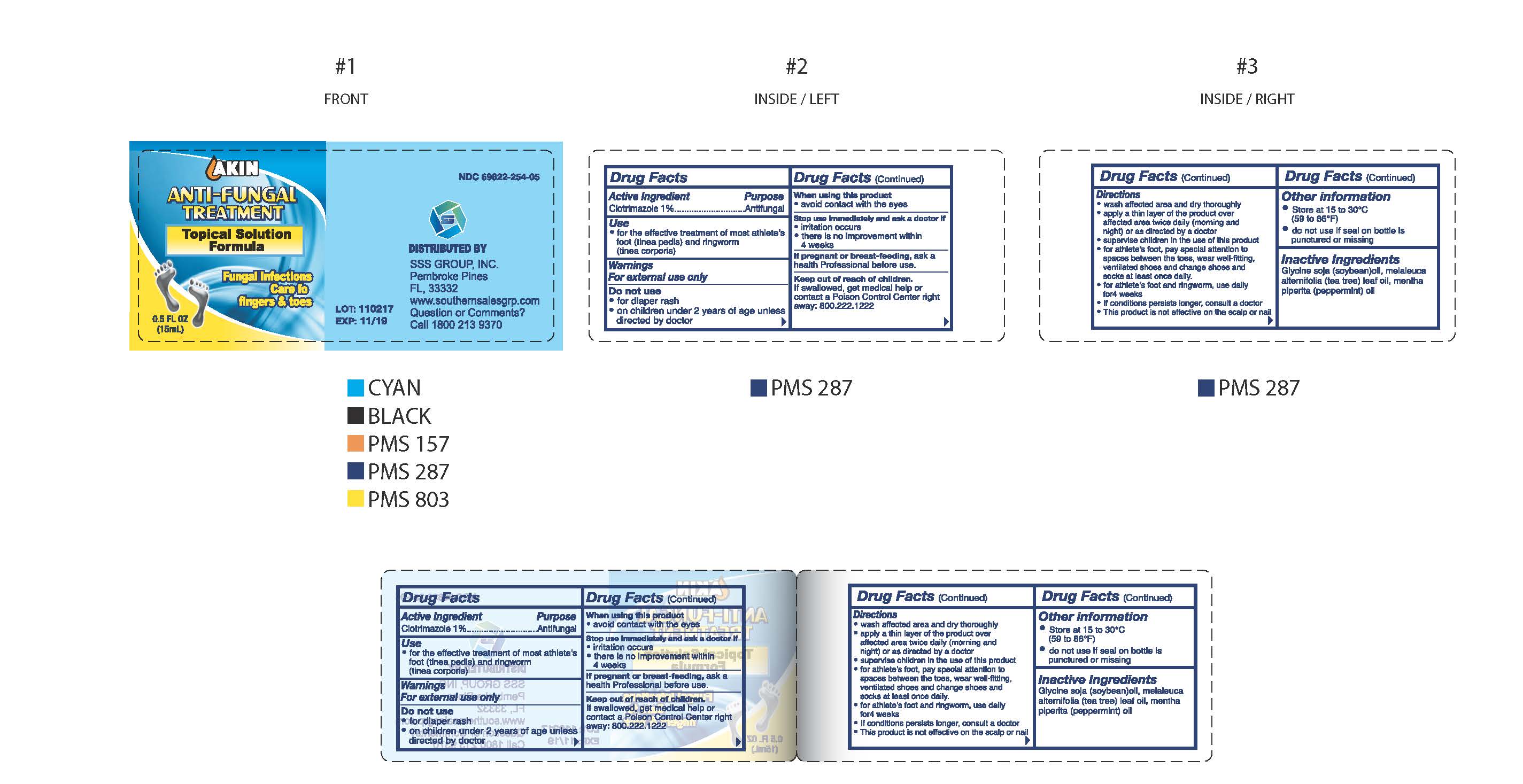
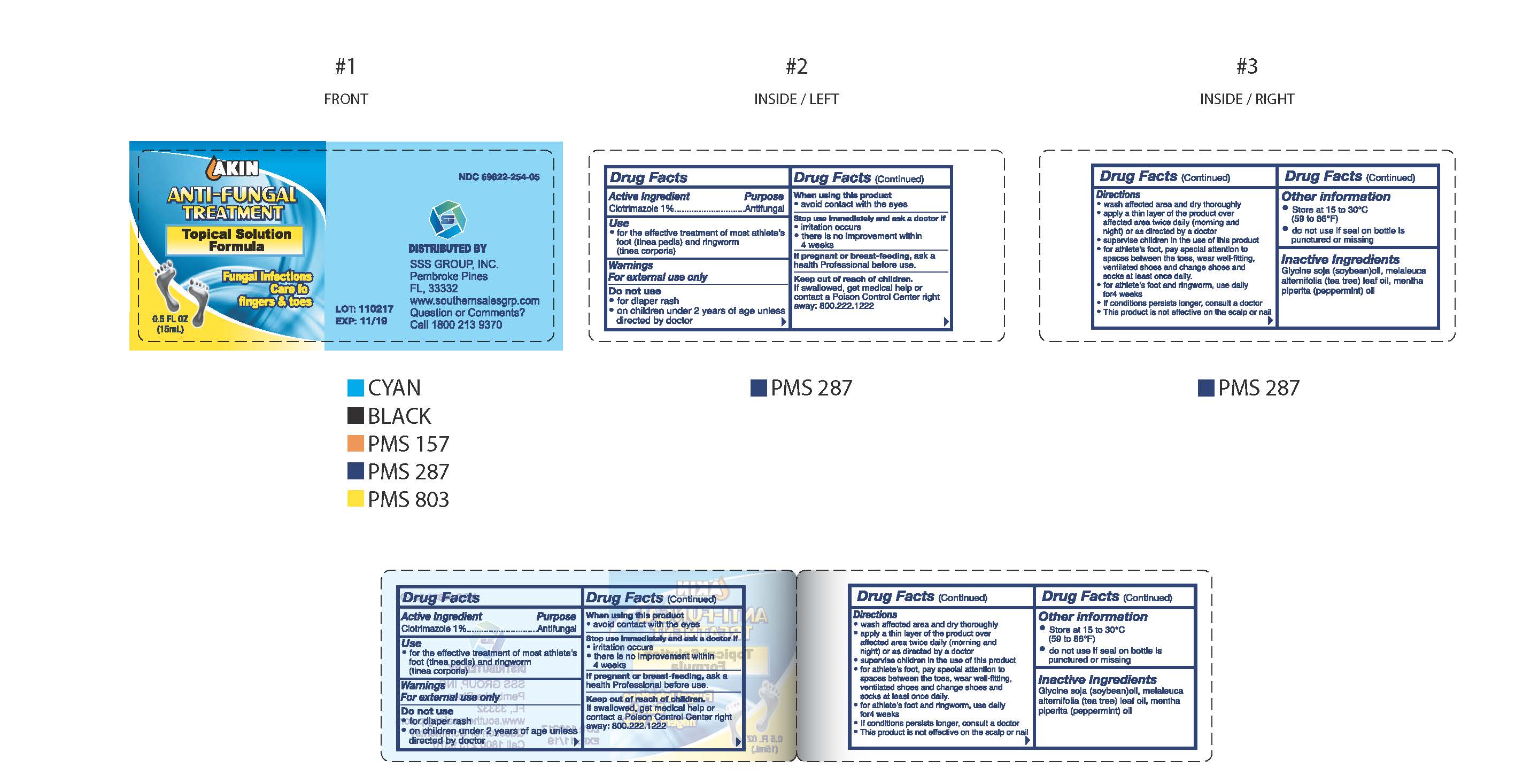
Label: AKIN ANTI-FUNGAL- clotrimazole solution
- NDC Code(s): 69822-254-05
- Packager: Southern Sales & Service, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 29, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
Drug Facts
Directions
- wash affected area and dry thoroughly
- apply a thin layer of the product over affeceted area twice daily (morning and night) or as directed by a doctor
- supervise children in the use of this product
- for athlete's foot, pay special attention to spaces between the toes, wear well-fitting, ventilated shoes and change shoes and socks at least once daily.
- for athlete's foot and ringworm, use daily for 4 weeks
- if condition persists longer, consult a doctor
- this product is not effective on the scalp or nails
- AKIN Anti-Fungal Treatment Topical Solution Formula 0.5oz (15 ml) 69822-254-05
-
INGREDIENTS AND APPEARANCE
AKIN ANTI-FUNGAL
clotrimazole solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69822-254 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CLOTRIMAZOLE (UNII: G07GZ97H65) (CLOTRIMAZOLE - UNII:G07GZ97H65) CLOTRIMAZOLE 10 mg in 1 mL Inactive Ingredients Ingredient Name Strength SOYBEAN OIL (UNII: 241ATL177A) MELALEUCA ALTERNIFOLIA LEAF (UNII: G43C57162K) MENTHA PIPERITA (UNII: 79M2M2UDA9) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69822-254-05 1 in 1 CARTON 01/01/2017 1 15 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M005 01/01/2017 Labeler - Southern Sales & Service, Inc. (013114906) Registrant - Southern Sales & Service, Inc. (013114906) Establishment Name Address ID/FEI Business Operations Southern Sales & Service, Inc. 013114906 label(69822-254) , manufacture(69822-254)