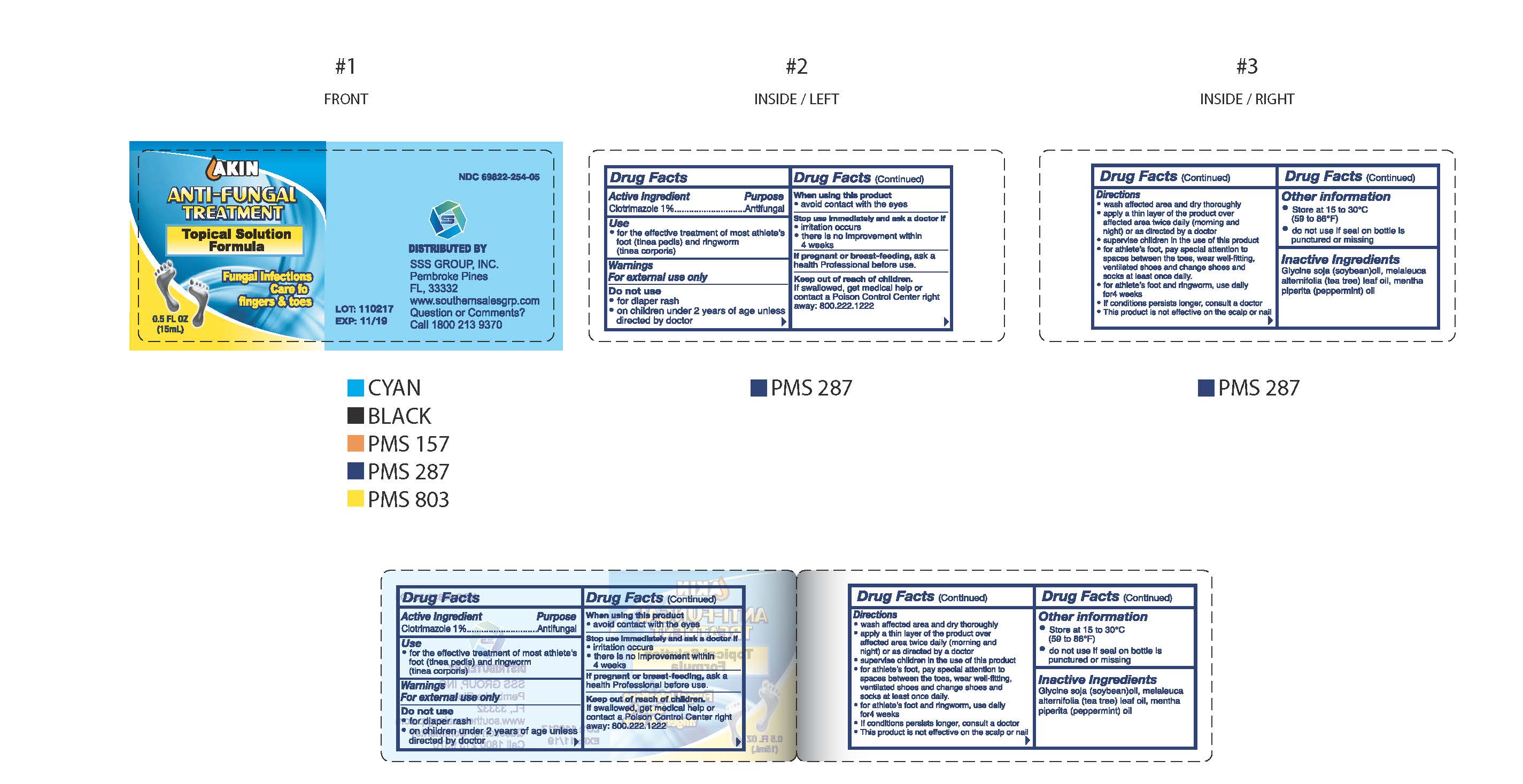
Drug Facts
Directions
- wash affected area and dry thoroughly
- apply a thin layer of the product over affeceted area twice daily (morning and night) or as directed by a doctor
- supervise children in the use of this product
- for athlete's foot, pay special attention to spaces between the toes, wear well-fitting, ventilated shoes and change shoes and socks at least once daily.
- for athlete's foot and ringworm, use daily for 4 weeks
- if condition persists longer, consult a doctor
- this product is not effective on the scalp or nails