Label: TATCHA THE SILK SUNSCREEN- zinc oxide lotion
- NDC Code(s): 69417-130-05, 69417-130-17
- Packager: TATCHA INC.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated March 28, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
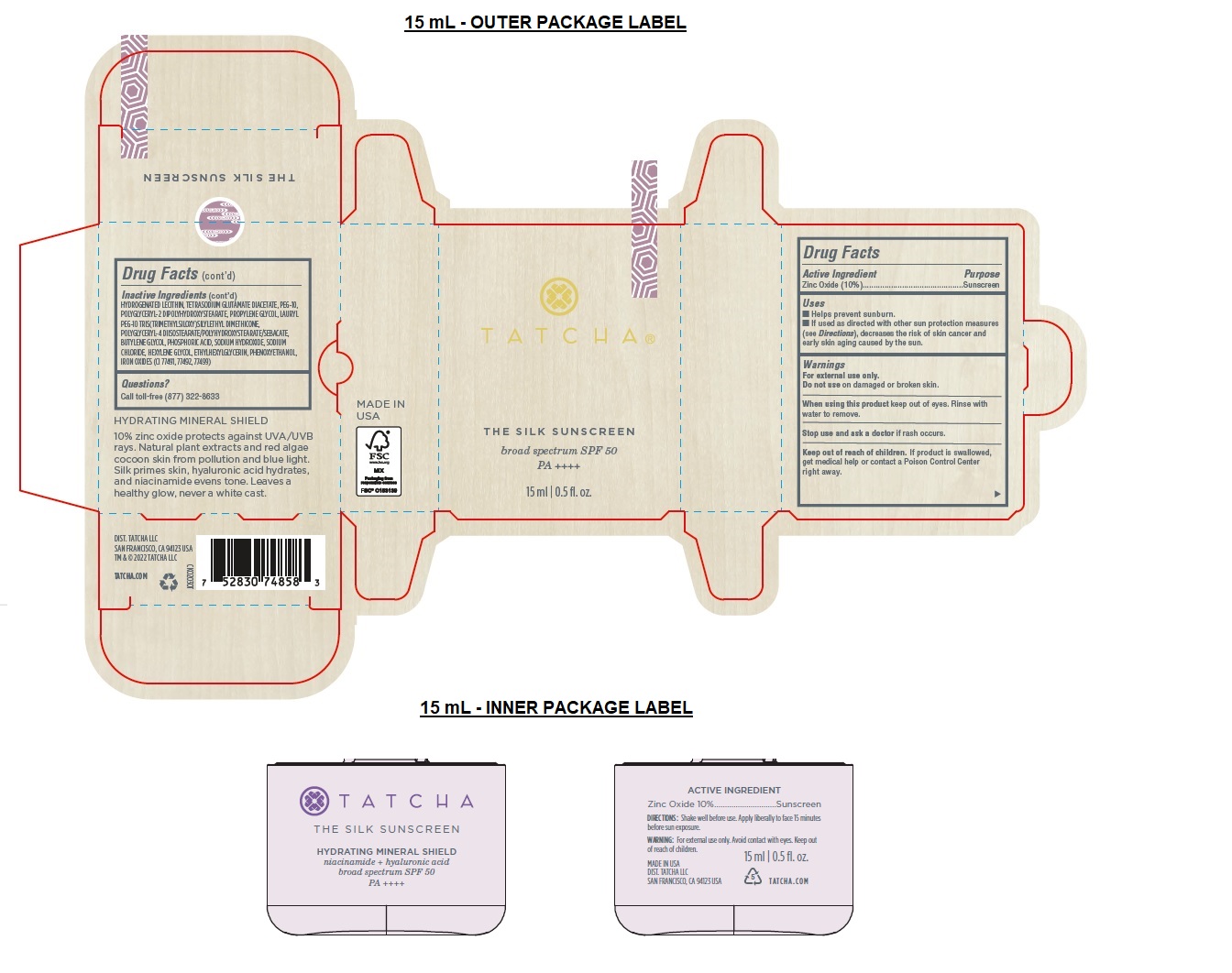
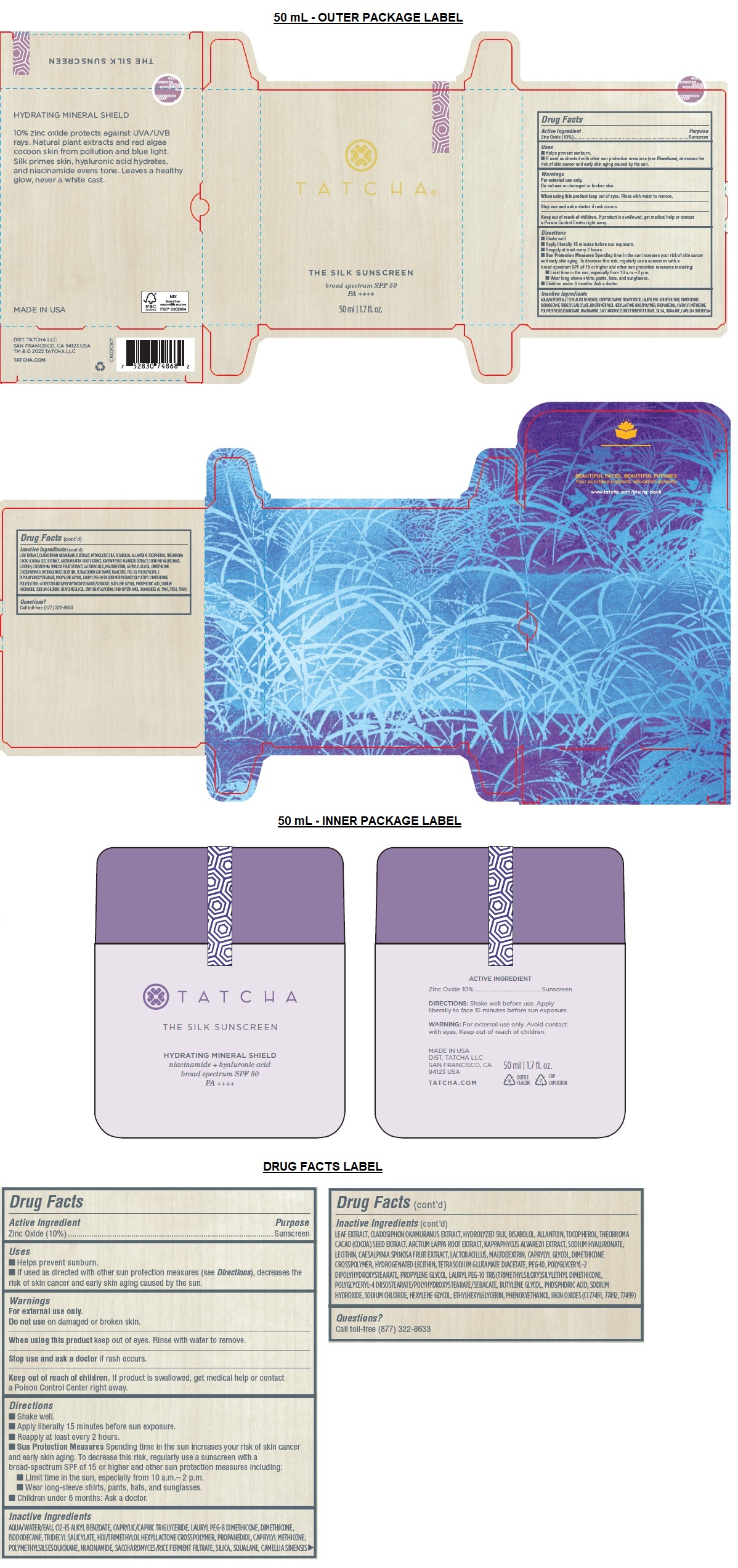
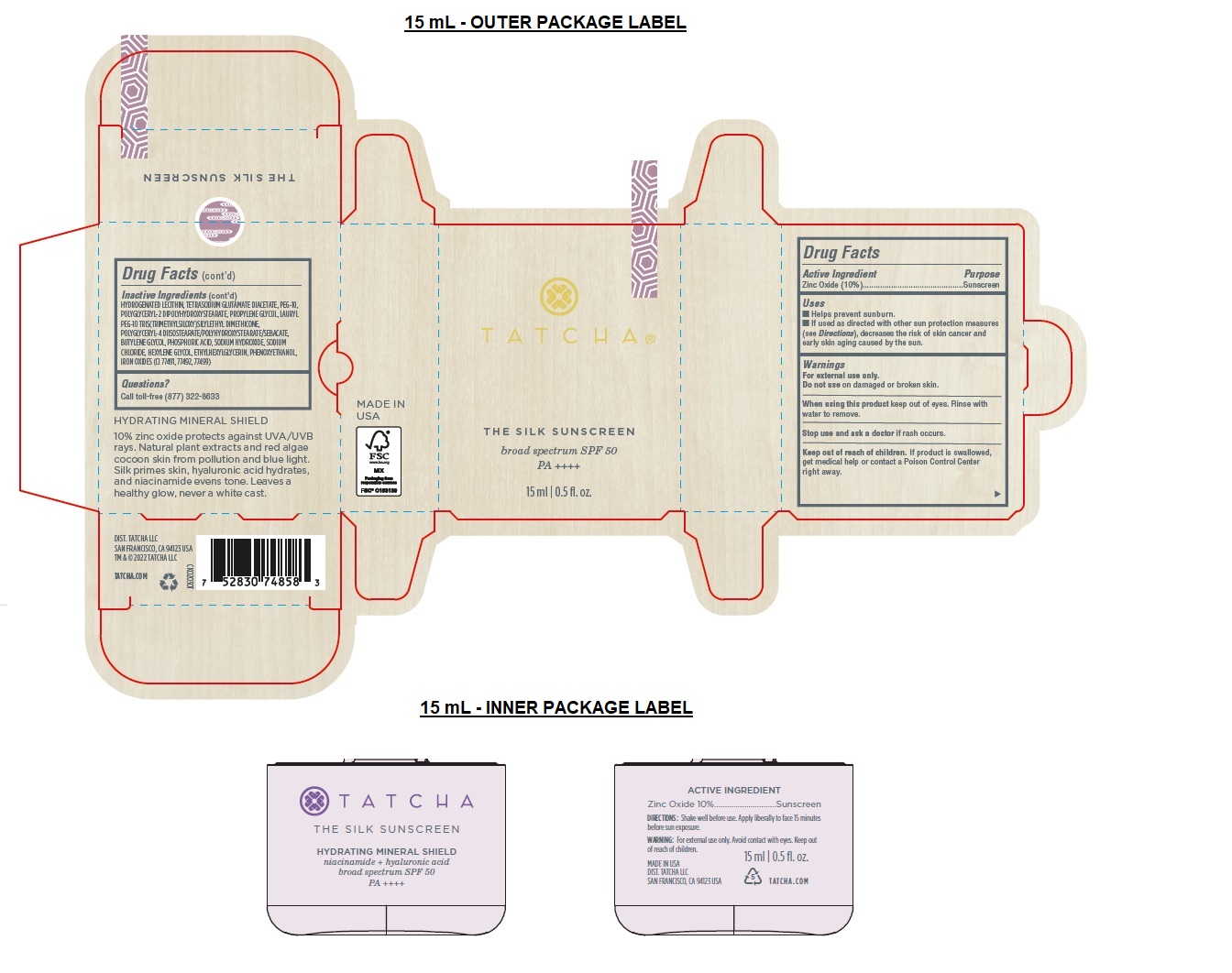
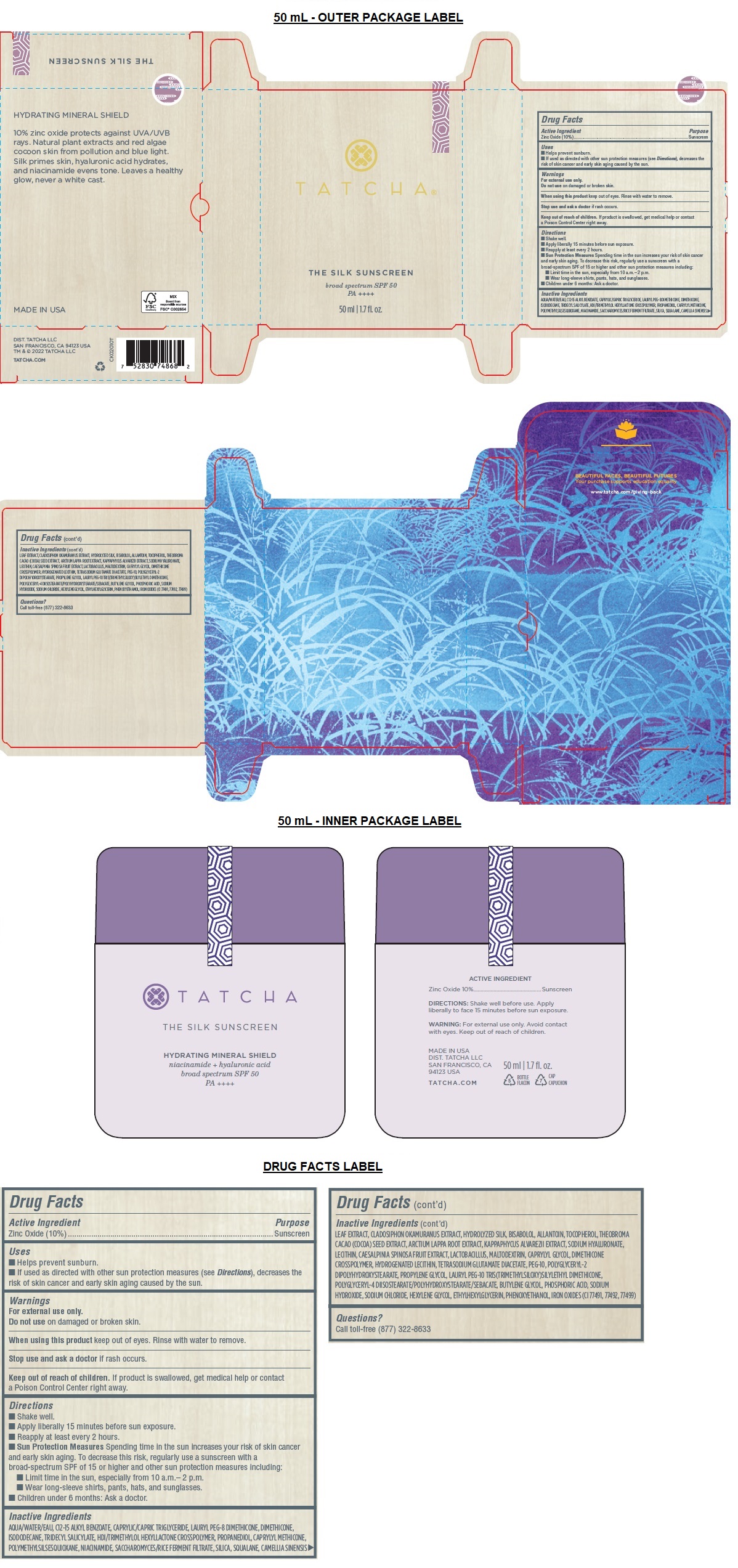
- Drug Facts
- Active Ingredient
- Purpose
- Uses
- Warnings
-
Directions
• Shake well.
• Apply liberally 15 minutes before sun exposure.
• Reapply at least every 2 hours.
• Sun Protection Measures Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a broad-spectrum SPF of 15 or higher and other sun protection measures including:
• Limit time in the sun, especially from 10 a.m.– 2 p.m.
• Wear long-sleeve shirts, pants, hats, and sunglasses.
• Children under 6 months: Ask a doctor. -
Inactive Ingredients
AQUA/WATER/EAU, C12-15 ALKYL BENZOATE, CAPRYLIC/CAPRIC TRIGLYCERIDE, LAURYL PEG-8 DIMETHICONE, DIMETHICONE, ISODODECANE, TRIDECYL SALICYLATE, HDI/TRIMETHYLOL HEXYLLACTONE CROSSPOLYMER, PROPANEDIOL, CAPRYLYL METHICONE, POLYMETHYLSILSESQUIOXANE, NIACINAMIDE, SACCHAROMYCES/RICE FERMENT FILTRATE, SILICA, SQUALANE, CAMELLIA SINENSIS LEAF EXTRACT, CLADOSIPHON OKAMURANUS EXTRACT, HYDROLYZED SILK, BISABOLOL, ALLANTOIN, TOCOPHEROL, THEOBROMA CACAO (COCOA) SEED EXTRACT, ARCTIUM LAPPA ROOT EXTRACT, KAPPAPHYCUS ALVAREZII EXTRACT, SODIUM HYALURONATE, LECITHIN, CAESALPINIA SPINOSA FRUIT EXTRACT, LACTOBACILLUS, MALTODEXTRIN, CAPRYLYL GLYCOL, DIMETHICONE CROSSPOLYMER, HYDROGENATED LECITHIN, TETRASODIUM GLUTAMATE DIACETATE, PEG-10, POLYGLYCERYL-2 DIPOLYHYDROXYSTEARATE, PROPYLENE GLYCOL, LAURYL PEG-10 TRIS(TRIMETHYLSILOXY)SILYLETHYL DIMETHICONE, POLYGLYCERYL-4 DIISOSTEARATE/POLYHYDROXYSTEARATE/SEBACATE, BUTYLENE GLYCOL, PHOSPHORIC ACID, SODIUM HYDROXIDE, SODIUM CHLORIDE, HEXYLENE GLYCOL, ETHYLHEXYLGLYCERIN, PHENOXYETHANOL, IRON OXIDES (CI 77491, 77492, 77499)
- Questions?
-
SPL UNCLASSIFIED SECTION
broad spectrum SPF 50
PA ++++
HYDRATING MINERAL SHIELD
10% zinc oxide protects against UVA/UVB rays. Natural plant extracts and red algae cocoon skin from pollution and blue light. Silk primes skin, hyaluronic acid hydrates, and niacinamide evens tone. Leaves a healthy glow, never a white cast.BEAUTIFUL FACES, BEAUTIFUL FUTURES
Your purchase supports education equality
www.tatcha.com/giving-backMADE IN USA
DIST. TATCHA LLC
SAN FRANCISCO, CA 94123 USA
TM & © 2022 TATCHA LLC
TATCHA.COM - Packaging
-
INGREDIENTS AND APPEARANCE
TATCHA THE SILK SUNSCREEN
zinc oxide lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69417-130 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 100 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) LAURYL PEG-8 DIMETHICONE (300 CPS) (UNII: ELL2U7K8T8) DIMETHICONE (UNII: 92RU3N3Y1O) ISODODECANE (UNII: A8289P68Y2) TRIDECYL SALICYLATE (UNII: AZQ08K38Z1) HEXAMETHYLENE DIISOCYANATE/TRIMETHYLOL HEXYLLACTONE CROSSPOLYMER (UNII: WB5K9Y35Y9) PROPANEDIOL (UNII: 5965N8W85T) CAPRYLYL TRISILOXANE (UNII: Q95M2P1KJL) POLYMETHYLSILSESQUIOXANE (4.5 MICRONS) (UNII: 59Z907ZB69) NIACINAMIDE (UNII: 25X51I8RD4) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SQUALANE (UNII: GW89575KF9) GREEN TEA LEAF (UNII: W2ZU1RY8B0) CLADOSIPHON OKAMURANUS (UNII: 2IJE0CH09J) SILK, BASE HYDROLYZED (1000 MW) (UNII: UMQ31C11AY) LEVOMENOL (UNII: 24WE03BX2T) ALLANTOIN (UNII: 344S277G0Z) TOCOPHEROL (UNII: R0ZB2556P8) COCOA (UNII: D9108TZ9KG) ARCTIUM LAPPA ROOT (UNII: 597E9BI3Z3) KAPPAPHYCUS ALVAREZII (UNII: T479H08K2O) HYALURONATE SODIUM (UNII: YSE9PPT4TH) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) CAESALPINIA SPINOSA FRUIT POD (UNII: EXY4496LWD) LACTOBACILLUS FERMENTUM (UNII: 2C1F12C6AP) MALTODEXTRIN (UNII: 7CVR7L4A2D) CAPRYLYL GLYCOL (UNII: 00YIU5438U) DIMETHICONE CROSSPOLYMER (450000 MPA.S AT 12% IN CYCLOPENTASILOXANE) (UNII: UF7620L1W6) HYDROGENATED SOYBEAN LECITHIN (UNII: H1109Z9J4N) TETRASODIUM GLUTAMATE DIACETATE (UNII: 5EHL50I4MY) POLYETHYLENE GLYCOL 500 (UNII: 761NX2Q08Y) POLYGLYCERYL-2 DIPOLYHYDROXYSTEARATE (UNII: 9229XJ4V12) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) POLYGLYCERYL-4 DIISOSTEARATE/POLYHYDROXYSTEARATE/SEBACATE (UNII: 687U3PEB2Y) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) PHOSPHORIC ACID (UNII: E4GA8884NN) SODIUM HYDROXIDE (UNII: 55X04QC32I) SODIUM CHLORIDE (UNII: 451W47IQ8X) HEXYLENE GLYCOL (UNII: KEH0A3F75J) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) PHENOXYETHANOL (UNII: HIE492ZZ3T) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69417-130-05 1 in 1 BOX 03/08/2022 1 15 mL in 1 BOTTLE; Type 0: Not a Combination Product 2 NDC:69417-130-17 1 in 1 BOX 03/08/2022 2 50 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part352 03/08/2022 Labeler - TATCHA INC. (006811461)