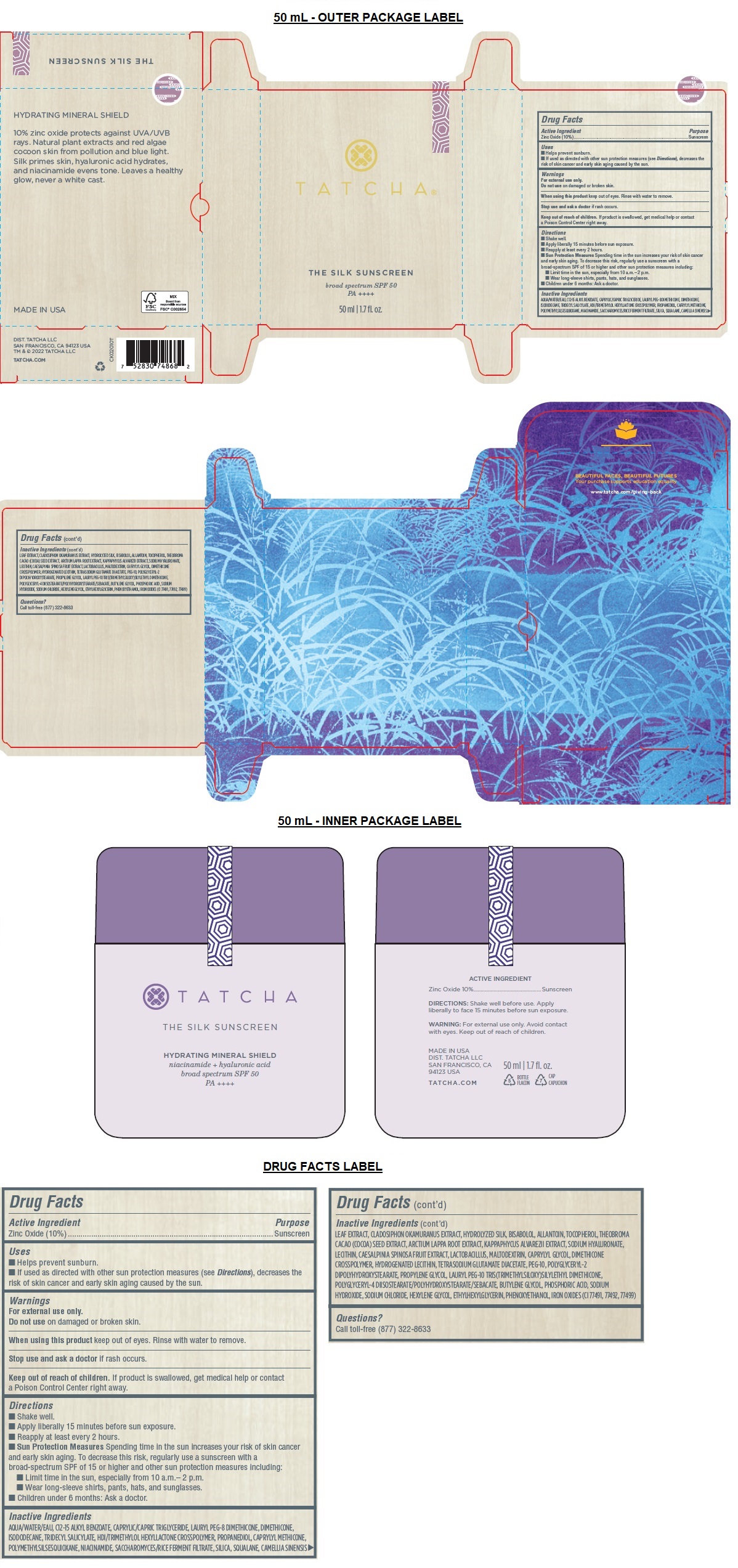
Uses
• Helps prevent sunburn.
• If used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun.
Warnings
For external use only.
Do not use on damaged or broken skin.
When using this product keep out of eyes. Rinse with water to remove.
Stop use and ask a doctor if rash occurs.
Directions
• Shake well.
• Apply liberally 15 minutes before sun exposure.
• Reapply at least every 2 hours.
• Sun Protection Measures Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a broad-spectrum SPF of 15 or higher and other sun protection measures including:
• Limit time in the sun, especially from 10 a.m.– 2 p.m.
• Wear long-sleeve shirts, pants, hats, and sunglasses.
• Children under 6 months: Ask a doctor.
Inactive Ingredients
AQUA/WATER/EAU, C12-15 ALKYL BENZOATE, CAPRYLIC/CAPRIC TRIGLYCERIDE, LAURYL PEG-8 DIMETHICONE, DIMETHICONE, ISODODECANE, TRIDECYL SALICYLATE, HDI/TRIMETHYLOL HEXYLLACTONE CROSSPOLYMER, PROPANEDIOL, CAPRYLYL METHICONE, POLYMETHYLSILSESQUIOXANE, NIACINAMIDE, SACCHAROMYCES/RICE FERMENT FILTRATE, SILICA, SQUALANE, CAMELLIA SINENSIS LEAF EXTRACT, CLADOSIPHON OKAMURANUS EXTRACT, HYDROLYZED SILK, BISABOLOL, ALLANTOIN, TOCOPHEROL, THEOBROMA CACAO (COCOA) SEED EXTRACT, ARCTIUM LAPPA ROOT EXTRACT, KAPPAPHYCUS ALVAREZII EXTRACT, SODIUM HYALURONATE, LECITHIN, CAESALPINIA SPINOSA FRUIT EXTRACT, LACTOBACILLUS, MALTODEXTRIN, CAPRYLYL GLYCOL, DIMETHICONE CROSSPOLYMER, HYDROGENATED LECITHIN, TETRASODIUM GLUTAMATE DIACETATE, PEG-10, POLYGLYCERYL-2 DIPOLYHYDROXYSTEARATE, PROPYLENE GLYCOL, LAURYL PEG-10 TRIS(TRIMETHYLSILOXY)SILYLETHYL DIMETHICONE, POLYGLYCERYL-4 DIISOSTEARATE/POLYHYDROXYSTEARATE/SEBACATE, BUTYLENE GLYCOL, PHOSPHORIC ACID, SODIUM HYDROXIDE, SODIUM CHLORIDE, HEXYLENE GLYCOL, ETHYLHEXYLGLYCERIN, PHENOXYETHANOL, IRON OXIDES (CI 77491, 77492, 77499)
broad spectrum SPF 50
PA ++++
HYDRATING MINERAL SHIELD
10% zinc oxide protects against UVA/UVB rays. Natural plant extracts and red algae cocoon skin from pollution and blue light. Silk primes skin, hyaluronic acid hydrates, and niacinamide evens tone. Leaves a healthy glow, never a white cast.
BEAUTIFUL FACES, BEAUTIFUL FUTURES
Your purchase supports education equality
www.tatcha.com/giving-back
MADE IN USA
DIST. TATCHA LLC
SAN FRANCISCO, CA 94123 USA
TM & © 2022 TATCHA LLC
TATCHA.COM