Label: FEXOFENADINE HYDROCHLORIDE AND PSEUDOEPHEDRINE HYDROCHLORIDE tablet, extended release
- NDC Code(s): 41415-995-20, 41415-995-30
- Packager: PUBLIX SUPER MARKETS, INC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated April 4, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
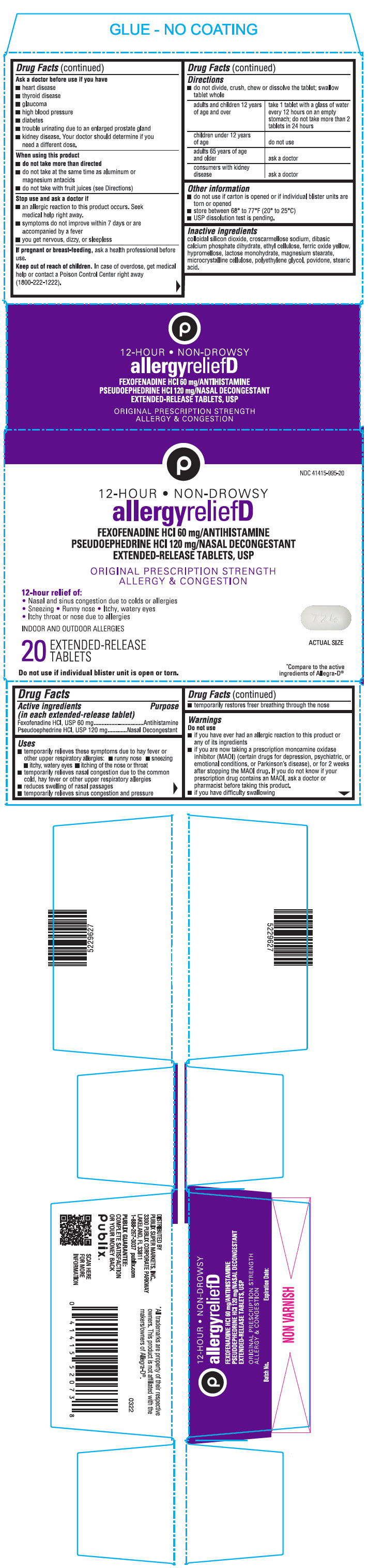
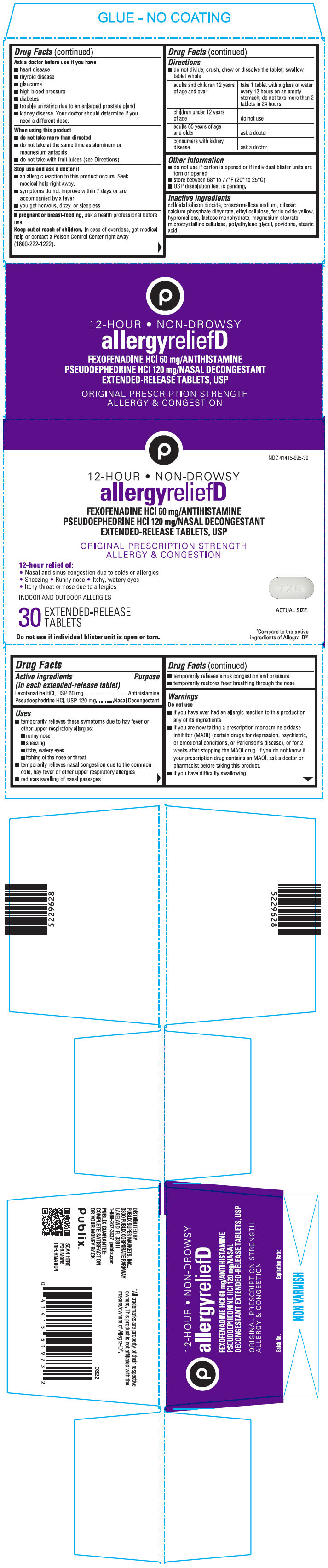
- Active ingredients (in each extended-release tablet)
- Purpose
-
Uses
- temporarily relieves these symptoms due to hay fever or other upper respiratory allergies:
- runny nose
- sneezing
- itchy, watery eyes
- itching of the nose or throat
- temporarily relieves nasal congestion due to the common cold, hay fever or other upper respiratory allergies
- reduces swelling of nasal passages
- temporarily relieves sinus congestion and pressure
- temporarily restores freer breathing through the nose
-
Warnings
Do not use
- if you have ever had an allergic reaction to this product or any of its ingredients
- if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
- if you have difficulty swallowing
Ask a doctor before use if you have
- heart disease
- thyroid disease
- glaucoma
- high blood pressure
- diabetes
- trouble urinating due to an enlarged prostate gland
- kidney disease. Your doctor should determine if you need a different dose.
When using this product
- do not take more than directed
- do not take at the same time as aluminum or magnesium antacids
- do not take with fruit juices (see Directions)
Stop use and ask a Doctor if
- an allergic reaction to this product occurs. Seek medical help right away.
- symptoms do not improve within 7 days or are accompanied by a fever
- you get nervous, dizzy, or sleepless
-
Directions
- do not divide, crush, chew or dissolve the tablet; swallow tablet whole
adults and children 12 years of age and over take 1 tablet with a glass of water every 12 hours on an empty stomach; do not take more than 2 tablets in 24 hours children under 12 years of age do not use adults 65 years of age and older ask a doctor consumers with kidney disease ask a doctor - Other information
- Inactive ingredients
- SPL UNCLASSIFIED SECTION
-
PRINCIPAL DISPLAY PANEL - 20 Tablet Blister Pack Carton
NDC 41415-995-20
12-HOUR • NON-DROWSY
allergyreliefD
FEXOFENADINE HCl 60 mg/ANTIHISTAMINE
PSEUDOEPHEDRINE HCl 120 mg/NASAL DECONGESTANT
EXTENDED-RELEASE TABLETS, USPORIGINAL PRESCRIPTION STRENGTH
ALLERGY & CONGESTION12-hour relief of:
• Nasal and sinus congestion due to colds or allergies
• Sneezing • Runny nose • Itchy, watery eyes
• Itchy throat or nose due to allergiesINDOOR AND OUTDOOR ALLERGIES
20
EXTENDED-RELEASE
TABLETSACTUAL SIZE
Do not use if individual blister unit is open or torn.
*Compare to the active
ingredients of Allegra-D®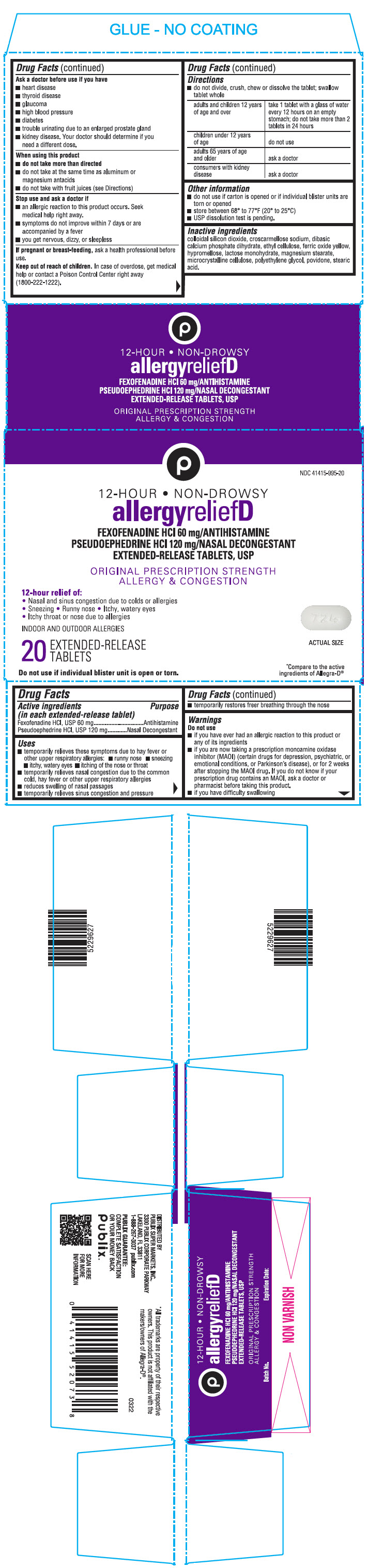
-
PRINCIPAL DISPLAY PANEL - 30 Tablet Blister Pack Carton
NDC 41415-995-30
12-HOUR • NON-DROWSY
allergyreliefD
FEXOFENADINE HCl 60 mg/ANTIHISTAMINE
PSEUDOEPHEDRINE HCl 120 mg/NASAL DECONGESTANT
EXTENDED-RELEASE TABLETS, USPORIGINAL PRESCRIPTION STRENGTH
ALLERGY & CONGESTION12-hour relief of:
• Nasal and sinus congestion due to colds or allergies
• Sneezing • Runny nose • Itchy, watery eyes
• Itchy throat or nose due to allergiesINDOOR AND OUTDOOR ALLERGIES
30
EXTENDED-RELEASE
TABLETSDo not use if individual blister unit is open or torn.
*Compare to the active
ingredients of Allegra-D®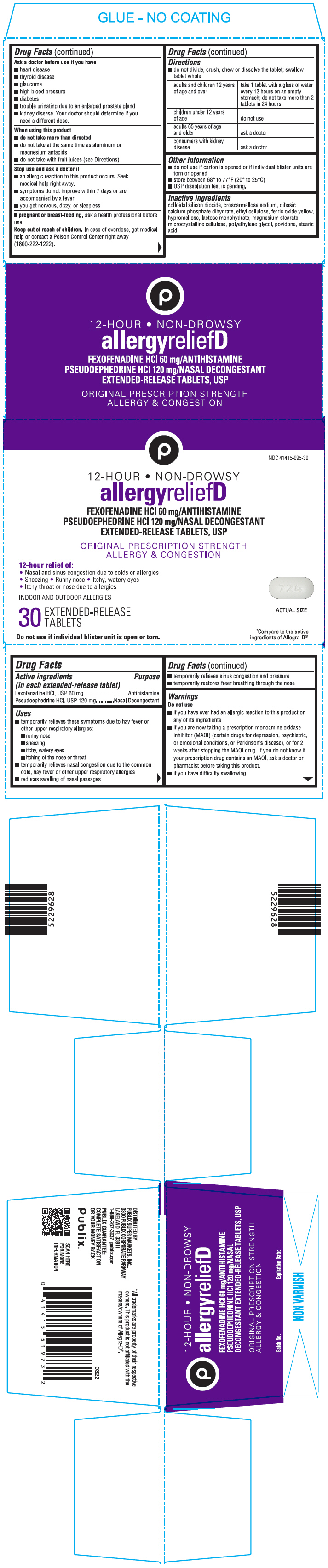
-
INGREDIENTS AND APPEARANCE
FEXOFENADINE HYDROCHLORIDE AND PSEUDOEPHEDRINE HYDROCHLORIDE
fexofenadine hydrochloride and pseudoephedrine hydrochloride tablet, extended releaseProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:41415-995 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FEXOFENADINE HYDROCHLORIDE (UNII: 2S068B75ZU) (FEXOFENADINE - UNII:E6582LOH6V) FEXOFENADINE HYDROCHLORIDE 60 mg PSEUDOEPHEDRINE HYDROCHLORIDE (UNII: 6V9V2RYJ8N) (PSEUDOEPHEDRINE - UNII:7CUC9DDI9F) PSEUDOEPHEDRINE HYDROCHLORIDE 120 mg Inactive Ingredients Ingredient Name Strength LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) POVIDONE, UNSPECIFIED (UNII: FZ989GH94E) MAGNESIUM STEARATE (UNII: 70097M6I30) DIBASIC CALCIUM PHOSPHATE DIHYDRATE (UNII: O7TSZ97GEP) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) ETHYLCELLULOSE, UNSPECIFIED (UNII: 7Z8S9VYZ4B) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) STEARIC ACID (UNII: 4ELV7Z65AP) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) Product Characteristics Color WHITE Score no score Shape OVAL Size 17mm Flavor Imprint Code 724 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:41415-995-20 1 in 1 CARTON 11/08/2021 1 20 in 1 BLISTER PACK; Type 0: Not a Combination Product 2 NDC:41415-995-30 1 in 1 CARTON 11/08/2021 2 30 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA090818 11/08/2021 Labeler - PUBLIX SUPER MARKETS, INC (006922009) Establishment Name Address ID/FEI Business Operations Sun Pharmaceutical Industries Limited 650445203 ANALYSIS(41415-995) , MANUFACTURE(41415-995)