Label: SYSTANE ULTRA PRESERVATIVE FREE- polyethylene glycol 400 and propylene glycol solution/ drops
- NDC Code(s): 0065-1507-82, 0065-1507-92
- Packager: Alcon Laboratories, Inc.
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated December 15, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- PURPOSE
- ACTIVE INGREDIENT
- Uses
- Warnings
- Do not use
- When using the product
- STOP USE
- Keep out of reach of children.
- Directions
- Other Information
- Inactive ingredients
- Questions?
-
Directions for Use:
- Hold the bottle just below the cap and twist the cap to open.
- BEFORE FIRST USE, hold the bottle upside down and dispense one drop of product to discard.
- Shake the bottle downward to remove residual product (see picture).
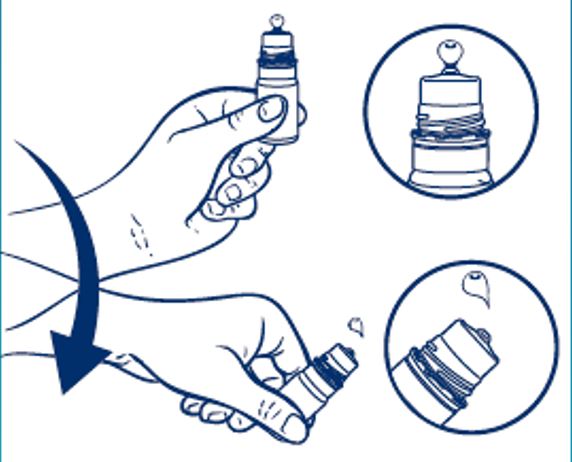
- Place 1 or 2 drops in the affected eye(s) as needed and blink.
- After use, shake the bottle downward to remove residual product that may be left on the tip (see picture above).
- Replace cap after use.
-
PRINCIPAL DISPLAY PANEL
Preservative-Free
Systane®
LUBRICANT EYE DROPS
ULTRA PF
PRESERVATIVE-FREE
DRY EYE RELIEF
Fast-acting hydration and lasting relief
Moisture rich formula for sensitive eyes
Preservative-Free Bottle
DOCTOR RECOMMENDED #1 Brand1
STERILE
10 mL (0.34 FL OZ)
Alcon
SIDE PANEL:
TAMPER EVIDENT:
For your protection, this bottle has a tamper evident ring around the bottom of the cap. Do not use if this ring is damaged or missing at the time of purchase.
1Based on a survey of eye care professionals. Data on file.
300059081-1022
Alcon Laboratories, Inc.
Fort Worth, TX 76134 USA
Country of Origin United States
www.alconpatents.com

PRINCIPAL DISPLAY PANEL
TWIN PACK
Preservative-Free
Systane®
LUBRICANT EYE DROPS
ULTRA PF
PRESERVATIVE-FREE
DRY EYE RELIEF
Fast-acting hydration and lasting relief
Moisture rich formula for sensitive eyes
Preservative-Free Bottles
DOCTOR RECOMMENDED #1 Brand1
Alcon
STERILE
Two 10 mL (0.34 FL OZ) Bottles
LOT: EXP.:
SIDE PANEL:
TAMPER EVIDENT:
For your protection, this bottle has a tamper evident ring around the bottom of the cap. Do not use if this ring is damaged or missing at the time of purchase.
1Based on a survey of eye care professionals. Data on file.
Alcon Laboratories, Inc.
Fort Worth, TX 76134 USA
Country of Origin USA
www.alconpatents.com
ACTUAL SIZE
300059122-1022

Systane®
LUBRICANT EYE DROPS
ULTRA PF
PRESERVATIVE-FREE
DRY EYE RELIEF
STERILE
10 mL (0.34 FL OZ)
Only for use in the eye.
Store at room temperature.
TAMPER EVIDENT: For your protection, this bottle has a tamper evident ring around the bottom of the cap. Do not use if this ring is damaged or missing at the time of purchase.
Alcon Laboratories, Inc.
Fort Worth, TX 76134 USA
See Carton for Directions For Use.
Country of Origin United States
LOT: EXP:
300049451-0721
-
INGREDIENTS AND APPEARANCE
SYSTANE ULTRA PRESERVATIVE FREE
polyethylene glycol 400 and propylene glycol solution/ dropsProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0065-1507 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Polyethylene Glycol 400 (UNII: B697894SGQ) (Polyethylene Glycol 400 - UNII:B697894SGQ) Polyethylene Glycol 400 4 mg in 1 mL Propylene Glycol (UNII: 6DC9Q167V3) (Propylene Glycol - UNII:6DC9Q167V3) Propylene Glycol 3 mg in 1 mL Inactive Ingredients Ingredient Name Strength Aminomethylpropanol (UNII: LU49E6626Q) Boric Acid (UNII: R57ZHV85D4) Guaraprolose (3500 Mpa.S At 1%) (UNII: 3A1I7376TC) Potassium Chloride (UNII: 660YQ98I10) Water (UNII: 059QF0KO0R) Sodium Chloride (UNII: 451W47IQ8X) Sorbitol (UNII: 506T60A25R) Hydrochloric Acid (UNII: QTT17582CB) Sodium Hydroxide (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0065-1507-82 1 in 1 CARTON 02/15/2022 1 10 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product 2 NDC:0065-1507-92 2 in 1 CARTON 04/11/2023 2 10 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M018 02/15/2022 Labeler - Alcon Laboratories, Inc. (008018525) Establishment Name Address ID/FEI Business Operations Alcon Research LLC 007672236 manufacture(0065-1507)



