Label: INFLAMYAR- arnica montana, bellis perennis, bryonia cretica subsp. dioica root, guaiacum officinale resin, ledum palustre twig, ruta graveolens whole, toxicodendron pubescens leaf, and viscum album fruit pellet
- NDC Code(s): 59469-172-10
- Packager: Pekana-Naturheilmittel GmbH
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated December 15, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- PURPOSE
- Ingredients
- INACTIVE INGREDIENT
- Indications
- Dosage
- Warning
- SPL UNCLASSIFIED SECTION
-
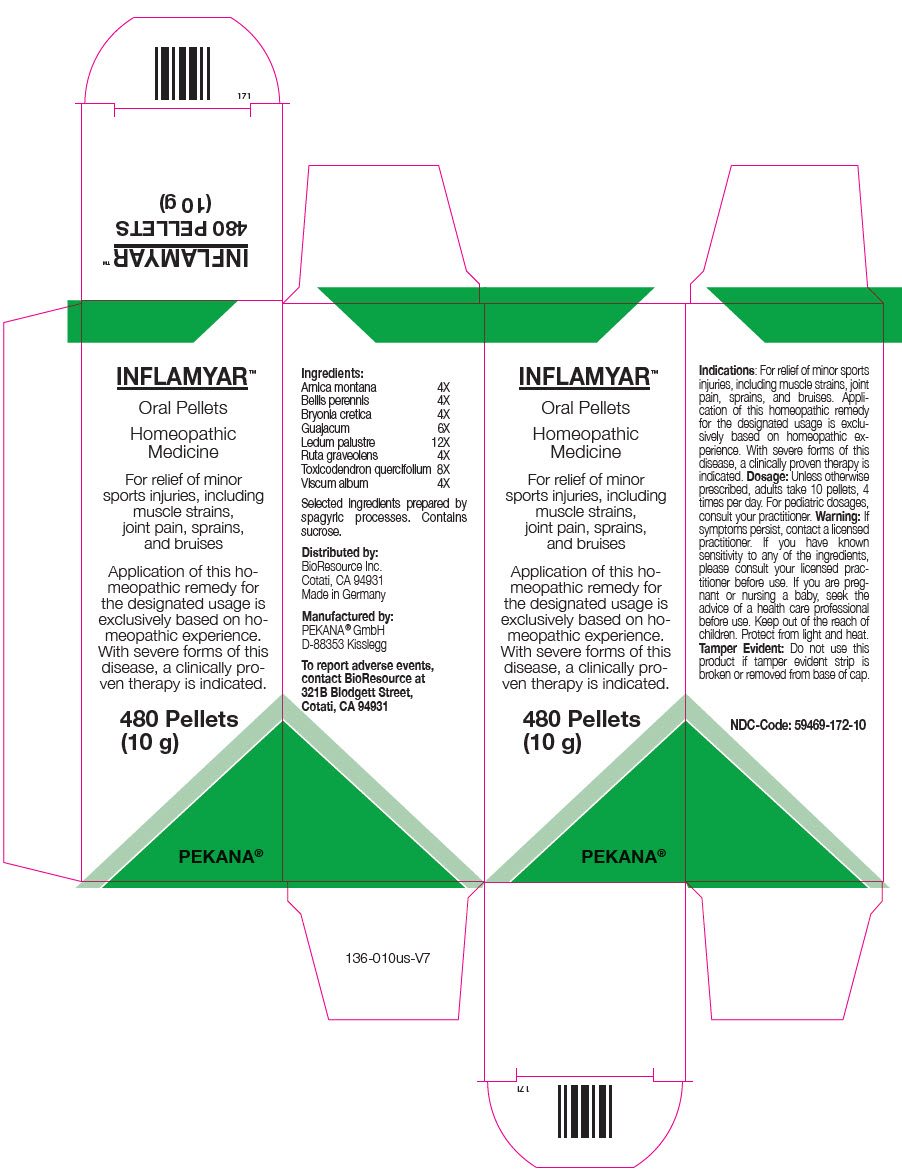
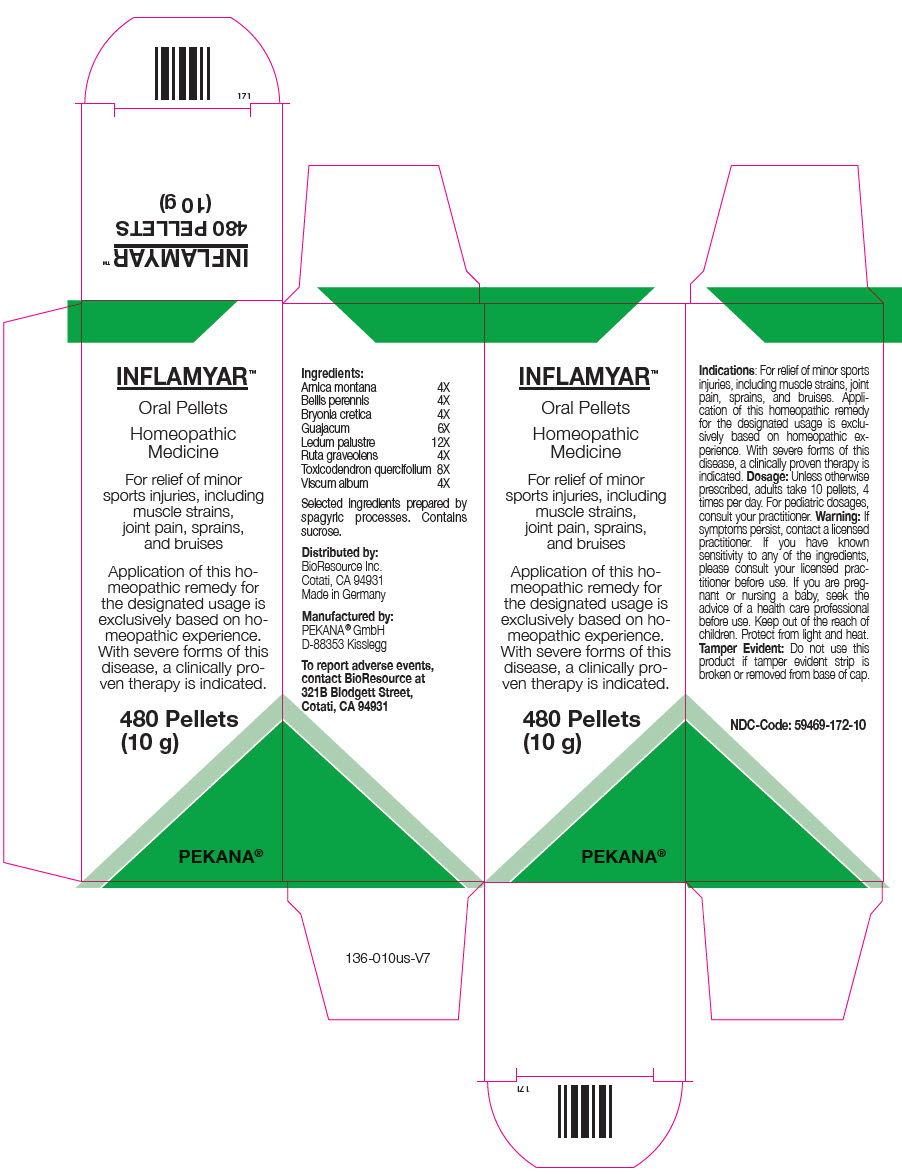
PRINCIPAL DISPLAY PANEL - 10 g Bottle Carton
INFLAMYAR™
Oral Pellets
Homeopathic
MedicineFor relief of minor
sports injuries, including
muscle strains,
joint pain, sprains,
and bruisesApplication of this ho-
meopathic remedy for
the designated usage is
exclusively based on ho-
meopathic experience.
With severe forms of this
disease, a clinically pro-
ven therapy is indicated.480 Pellets
(10 g)PEKANA®
-
INGREDIENTS AND APPEARANCE
INFLAMYAR
arnica montana, bellis perennis, bryonia cretica subsp. dioica root, guaiacum officinale resin, ledum palustre twig, ruta graveolens whole, toxicodendron pubescens leaf, and viscum album fruit pelletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:59469-172 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ARNICA MONTANA WHOLE (UNII: O80TY208ZW) (ARNICA MONTANA WHOLE - UNII:O80TY208ZW) ARNICA MONTANA WHOLE 4 [hp_X] BELLIS PERENNIS WHOLE (UNII: 2HU33I03UY) (BELLIS PERENNIS WHOLE - UNII:2HU33I03UY) BELLIS PERENNIS WHOLE 4 [hp_X] BRYONIA DIOICA ROOT (UNII: 53UB5FH7CX) (BRYONIA DIOICA ROOT - UNII:53UB5FH7CX) BRYONIA DIOICA ROOT 4 [hp_X] GUAIACUM OFFICINALE RESIN (UNII: N0K2Z502R6) (GUAIACUM OFFICINALE RESIN - UNII:N0K2Z502R6) GUAIACUM OFFICINALE RESIN 6 [hp_X] RHODODENDRON TOMENTOSUM LEAFY TWIG (UNII: 877L01IZ0P) (RHODODENDRON TOMENTOSUM LEAFY TWIG - UNII:877L01IZ0P) RHODODENDRON TOMENTOSUM LEAFY TWIG 12 [hp_X] RUTA GRAVEOLENS WHOLE (UNII: 181JI0338P) (RUTA GRAVEOLENS WHOLE - UNII:181JI0338P) RUTA GRAVEOLENS WHOLE 4 [hp_X] TOXICODENDRON PUBESCENS LEAF (UNII: 6IO182RP7A) (TOXICODENDRON PUBESCENS LEAF - UNII:6IO182RP7A) TOXICODENDRON PUBESCENS LEAF 8 [hp_X] VISCUM ALBUM FRUIT (UNII: P83EQ521R3) (VISCUM ALBUM FRUIT - UNII:P83EQ521R3) VISCUM ALBUM FRUIT 4 [hp_X] Inactive Ingredients Ingredient Name Strength SUCROSE (UNII: C151H8M554) Product Characteristics Color WHITE Score no score Shape ROUND Size 3mm Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59469-172-10 1 in 1 CARTON 09/01/2011 1 480 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date UNAPPROVED HOMEOPATHIC 09/01/2011 Labeler - Pekana-Naturheilmittel GmbH (320344542) Establishment Name Address ID/FEI Business Operations Pekana-Naturheilmittel GmbH 320344542 MANUFACTURE(59469-172)