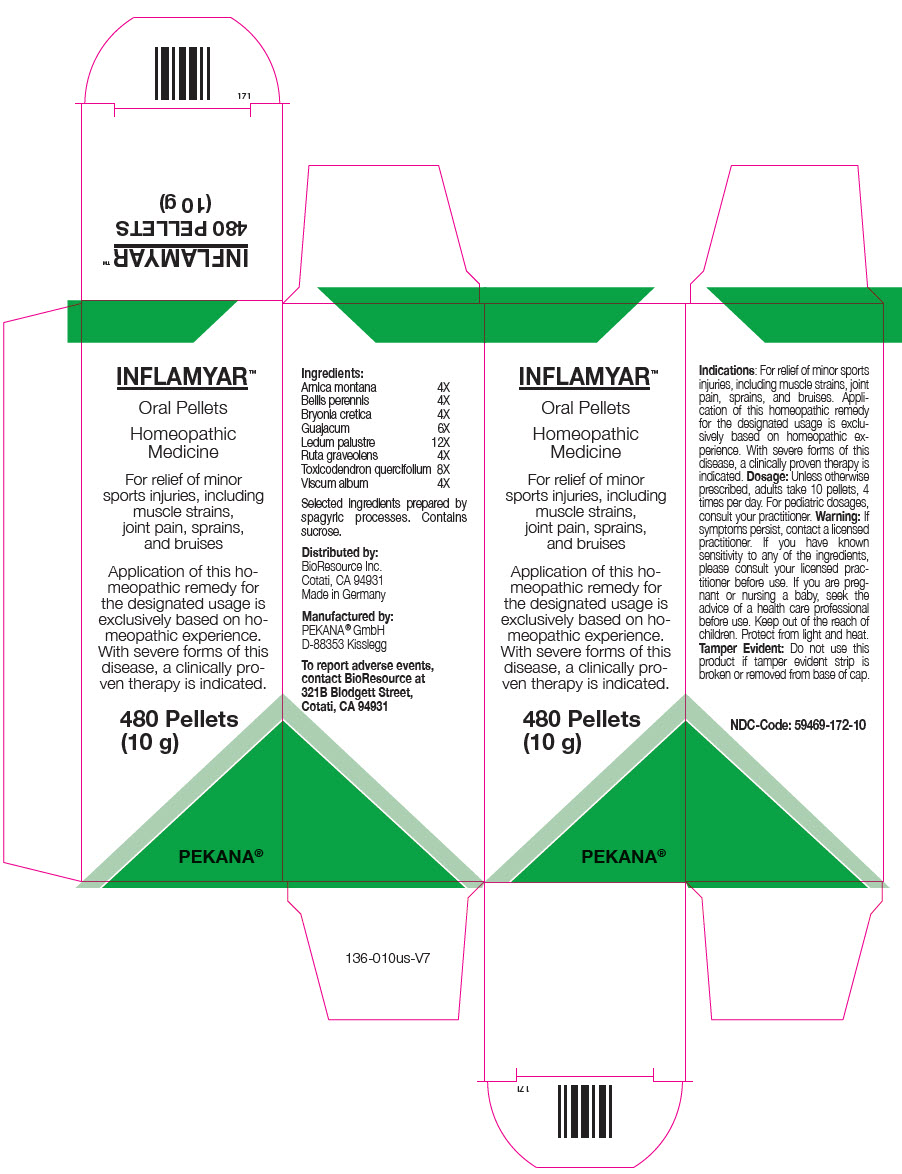
Ingredients
| Arnica montana | 4X |
| Bellis perennis | 4X |
| Bryonia cretica | 4X |
| Guajacum | 6X |
| Ledum palustre | 12X |
| Ruta graveolens | 4X |
| Toxicodendron quercifolium | 8X |
| Viscum album | 4X |
Indications
For relief of minor sports injuries, including muscle strains, joint pain, sprains, and bruises. Application of this homeopathic remedy for the designated usage is exclusively based on homeopathic experience. With severe forms of this disease, a clinically proven therapy is indicated.
Dosage
Unless otherwise prescribed, adults take 10 pellets, 4 times per day. For pediatric dosages, consult your practitioner.
Warning
If symptoms persist, contact a licensed practitioner. If you have known sensitivity to any of the ingredients, please consult your licensed practitioner before use. If you are pregnant or nursing a baby, seek the advice of a health care professional before use.
To report adverse events, contact BioResource at 321B Blodgett Street, Cotati, CA 94931
Manufactured by:
PEKANA® GmbH
D-88353 Kisslegg
Distributed by:
BioResource Inc.
Cotati, CA 94931
Made in Germany
PRINCIPAL DISPLAY PANEL - 10 g Bottle Carton
INFLAMYAR™
Oral Pellets
Homeopathic
Medicine
For relief of minor
sports injuries, including
muscle strains,
joint pain, sprains,
and bruises
Application of this ho-
meopathic remedy for
the designated usage is
exclusively based on ho-
meopathic experience.
With severe forms of this
disease, a clinically pro-
ven therapy is indicated.
480 Pellets
(10 g)
PEKANA®