Label: METADERM ECZEMA AND BABY ECZEMA CREAM- achillea millefolium, aesculus hippocastanum, berberis vulgaris, conium maculatum, matricaria chamomilla, phytolacca decandra, rhus toxicodendron, sanguinaria canadensis cream
-
Contains inactivated NDC Code(s)
NDC Code(s): 58133-360-06, 58133-360-32, 58133-360-65 - Packager: Cosmetic Specialty Labs, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated August 15, 2018
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
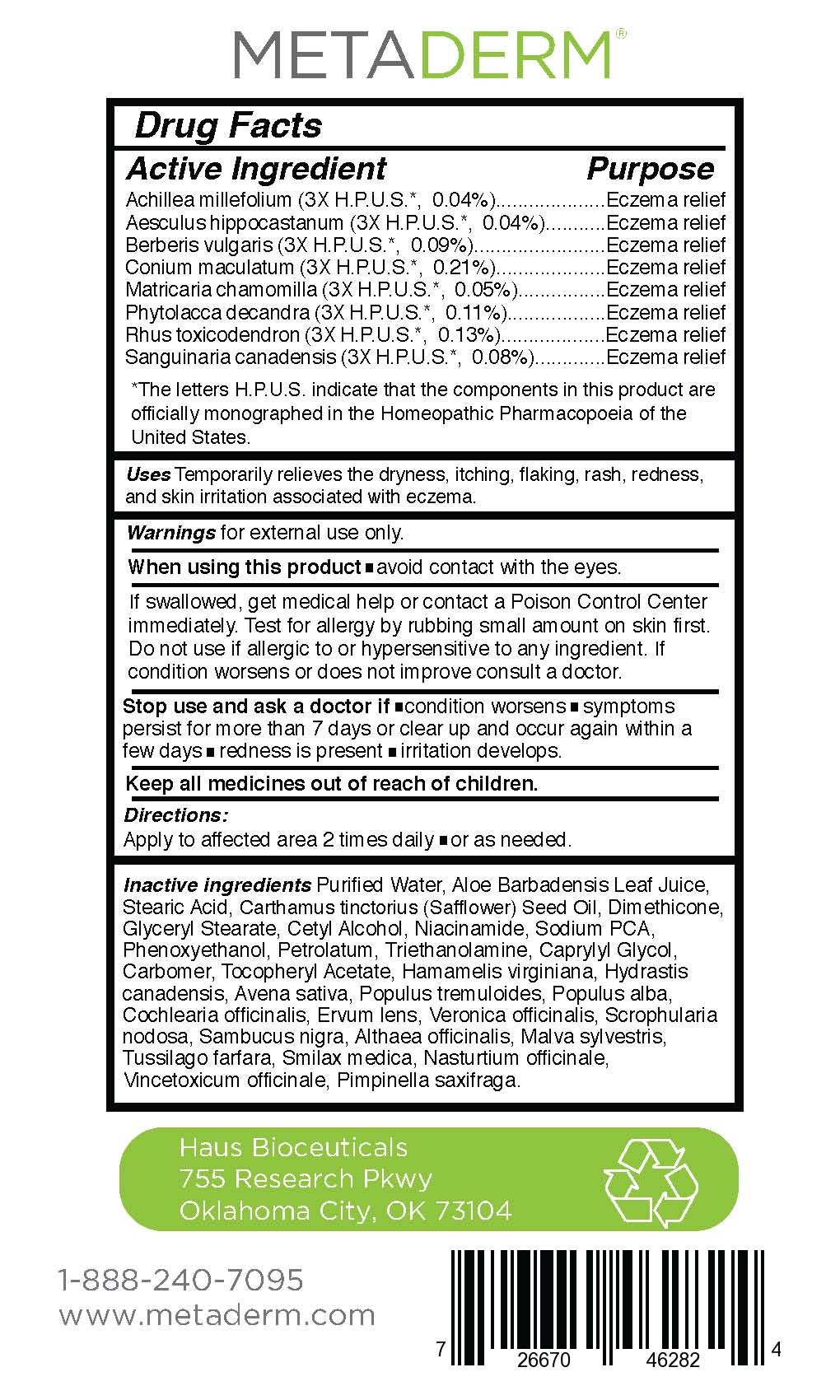
Active Ingredient
Achillea millefolium (3X H.P.U.S.*, 0.04%)
Aesculus hippocastanum (3X H.P.U.S.*, 0.04%)
Berberis vulgaris (3X H.P.U.S.*, 0.09%)
Conium maculatum (3X H.P.U.S.*, 0.21%)
Matricaria chamomilla (3X H.P.U.S.*, 0.05%)
Phytolacca decandra (3X H.P.U.S.*, 0.11%)
Rhus toxicodendron (3X H.P.U.S.*, 0.13%)
Sanguinaria canadensis (3X H.P.U.S.*, 0.08%) - Purpose
- Uses:
- Warnings:
- When using this product:
- Stop use and ask a doctor if:
- KEEP OUT OF REACH OF CHILDREN
- Directions:
-
Other ingredients:
Purified Water, Aloe Barbadensis Leaf Juice,
Stearic Acid, Carthamus tinctorius (Safflower) Seed Oil, Dimethicone,
Glyceryl Stearate, Cetyl Alcohol, Niacinamide, Sodium PCA,
Phenoxyethanol, Petrolatum, Triethanolamine, Caprylyl Glycol,
Carbomer, Tocopheryl Acetate, Hamamelis virginiana, Hydrastis
canadensis, Avena sativa, Populus tremuloides, Populus alba,
Cochlearia officinalis, Ervum lens, Veronica officinalis, Scrophularia
nodosa, Sambucus nigra, Althaea officinalis, Malva sylvestris,
Tussilago farfara, Smilax medica, Nasturtium officinale,
Vincetoxicum officinale, Pimpinella saxifraga. - Principal Display Panel and Drug Facts
-
INGREDIENTS AND APPEARANCE
METADERM ECZEMA AND BABY ECZEMA CREAM
achillea millefolium, aesculus hippocastanum, berberis vulgaris, conium maculatum, matricaria chamomilla, phytolacca decandra, rhus toxicodendron, sanguinaria canadensis creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:58133-360 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MATRICARIA CHAMOMILLA FLOWERING TOP OIL (UNII: SA8AR2W4ER) (MATRICARIA CHAMOMILLA FLOWERING TOP OIL - UNII:SA8AR2W4ER) MATRICARIA CHAMOMILLA FLOWERING TOP OIL 5 [hp_M] in 1 mL SANGUINARIA CANADENSIS ROOT (UNII: N9288CD508) (SANGUINARIA CANADENSIS ROOT - UNII:N9288CD508) SANGUINARIA CANADENSIS ROOT 8 [hp_M] in 1 mL ACHILLEA MILLEFOLIUM (UNII: 2FXJ6SW4PK) (ACHILLEA MILLEFOLIUM - UNII:2FXJ6SW4PK) ACHILLEA MILLEFOLIUM 4 [hp_M] in 1 mL BERBERIS VULGARIS FRUIT (UNII: 6XEF22AHC3) (BERBERIS VULGARIS FRUIT - UNII:6XEF22AHC3) BERBERIS VULGARIS FRUIT 9 [hp_M] in 1 mL CONIUM MACULATUM FRUIT (UNII: Y71WKJ3A2K) (CONIUM MACULATUM FRUIT - UNII:Y71WKJ3A2K) CONIUM MACULATUM FRUIT 21 [hp_M] in 1 mL RHUS SPP. WHOLE (UNII: 3U7FG9T9MW) (RHUS SPP. WHOLE - UNII:3U7FG9T9MW) RHUS SPP. WHOLE 13 [hp_M] in 1 mL PHYTOLACCA OCTANDRA LEAF (UNII: 0804KD28Q9) (PHYTOLACCA OCTANDRA LEAF - UNII:0804KD28Q9) PHYTOLACCA OCTANDRA LEAF 11 [hp_M] in 1 mL AESCULUS HIPPOCASTANUM SEED OIL (UNII: E0M52HIR1Y) (AESCULUS HIPPOCASTANUM SEED OIL - UNII:E0M52HIR1Y) AESCULUS HIPPOCASTANUM SEED OIL 4 [hp_M] in 1 mL Inactive Ingredients Ingredient Name Strength CARTHAMUS TINCTORIUS (SAFFLOWER) OLEOSOMES (UNII: 9S60Q72309) VERONICA OFFICINALIS LEAF (UNII: 96R87REA55) ALTHAEA OFFICINALIS LEAF (UNII: E2QQV92338) SCROPHULARIA NODOSA (UNII: 7H443NUB2T) WATER (UNII: 059QF0KO0R) ALPHA-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) TRIETHANOLAMINE LAURYLAMINOPROPIONATE (UNII: 793J74ICPW) ALOE VERA LEAF (UNII: ZY81Z83H0X) CETYL ALCOHOL (UNII: 936JST6JCN) HAMAMELIS VIRGINIANA ROOT BARK/STEM BARK (UNII: T7S323PKJS) PHENOXYETHANOL (UNII: HIE492ZZ3T) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CARBOMER 940 (UNII: 4Q93RCW27E) DIMETHICONE (UNII: 92RU3N3Y1O) HYDRASTIS CANADENSIS WHOLE (UNII: R763EBH88T) LENS CULINARIS FRUIT (UNII: ZYZ076G9JH) SAMBUCUS NIGRA FLOWER OIL (UNII: Q35633V53D) MALVA SYLVESTRIS LEAF (UNII: 17H39B00T5) TUSSILAGO FARFARA (UNII: 0JXZ63016V) STEARIC ACID (UNII: 4ELV7Z65AP) NIACINAMIDE (UNII: 25X51I8RD4) SODIUM PYRROLIDONE CARBOXYLATE (UNII: 469OTG57A2) AVENA SATIVA LEAF (UNII: 206PI19V7R) POPULUS TREMULOIDES LEAF (UNII: 7IIH57D9E0) POPULUS ALBA LEAF (UNII: A74CT64437) COCHLEARIA OFFICINALIS LEAF (UNII: WAG1VW36J6) SARSAPARILLA (UNII: 2H1576D5WG) NASTURTIUM OFFICINALE (UNII: YH89GMV676) VINCETOXICUM ATRATUM ROOT (UNII: 7DQZ24B35Y) PIMPINELLA SAXIFRAGA ROOT (UNII: 5Y05905N7G) GLYCERYL STEARATE SE (UNII: FCZ5MH785I) PETROLATUM (UNII: 4T6H12BN9U) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58133-360-32 950 mL in 1 BOTTLE; Type 0: Not a Combination Product 08/15/2018 2 NDC:58133-360-06 193 mL in 1 BOTTLE; Type 0: Not a Combination Product 08/15/2018 3 NDC:58133-360-65 193 mL in 1 BOTTLE; Type 0: Not a Combination Product 08/15/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 08/15/2018 Labeler - Cosmetic Specialty Labs, Inc. (032973000) Registrant - Cosmetic Specialty Labs, Inc. (032973000) Establishment Name Address ID/FEI Business Operations Cosmetic Specialty Labs, Inc. 032973000 manufacture(58133-360)