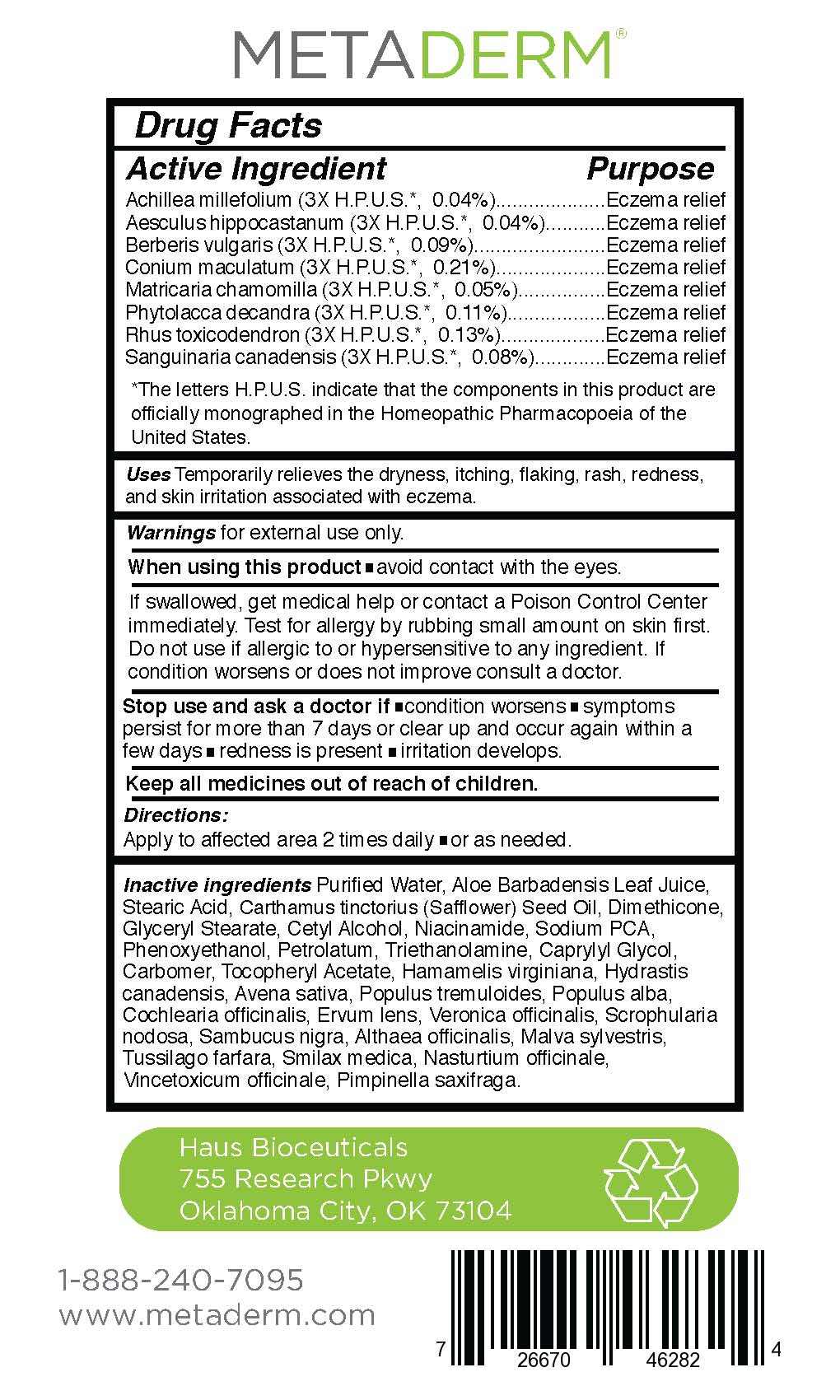
Active Ingredient
Achillea millefolium (3X H.P.U.S.*, 0.04%)
Aesculus hippocastanum (3X H.P.U.S.*, 0.04%)
Berberis vulgaris (3X H.P.U.S.*, 0.09%)
Conium maculatum (3X H.P.U.S.*, 0.21%)
Matricaria chamomilla (3X H.P.U.S.*, 0.05%)
Phytolacca decandra (3X H.P.U.S.*, 0.11%)
Rhus toxicodendron (3X H.P.U.S.*, 0.13%)
Sanguinaria canadensis (3X H.P.U.S.*, 0.08%)
Purpose
Eczema relief
*The letters H.P.U.S. indicate that the components in this product are officially monographed in the Homeopathic Pharmacopoeia of the United States.
Uses:
Temporarily relieves the dryness, itching, flaking, rash, redness,
and skin irritation associated with eczema.
When using this product:
- Avoid contact with eyes.
- If swallowed, get medical help or contact a Poison Control Center immediately.
- Test for allergy by rubbing small amount on skin first.
- Do not use if allergic to or hypersensitive to any ingredient.
- If condition worsens or does not improve consult a doctor.
Stop use and ask a doctor if:
- condition worsens
- symptoms persist for more than 7 days or clear up and occur again within a few days
- redness is present
- irritation develops
Other ingredients:
Purified Water, Aloe Barbadensis Leaf Juice,
Stearic Acid, Carthamus tinctorius (Safflower) Seed Oil, Dimethicone,
Glyceryl Stearate, Cetyl Alcohol, Niacinamide, Sodium PCA,
Phenoxyethanol, Petrolatum, Triethanolamine, Caprylyl Glycol,
Carbomer, Tocopheryl Acetate, Hamamelis virginiana, Hydrastis
canadensis, Avena sativa, Populus tremuloides, Populus alba,
Cochlearia officinalis, Ervum lens, Veronica officinalis, Scrophularia
nodosa, Sambucus nigra, Althaea officinalis, Malva sylvestris,
Tussilago farfara, Smilax medica, Nasturtium officinale,
Vincetoxicum officinale, Pimpinella saxifraga.