Label: ABSORBINE JR. PLUS PAIN RELIEF MICRO- camphor, menthol patch
- NDC Code(s): 69693-415-01
- Packager: Clarion Brands, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 21, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- INDICATIONS & USAGE
- Warnings
- When using this product
- Stop use and ask a doctor if
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
- DOSAGE & ADMINISTRATION
- STORAGE AND HANDLING
- INACTIVE INGREDIENT
- Questions? Call 1-844-922-7672
-
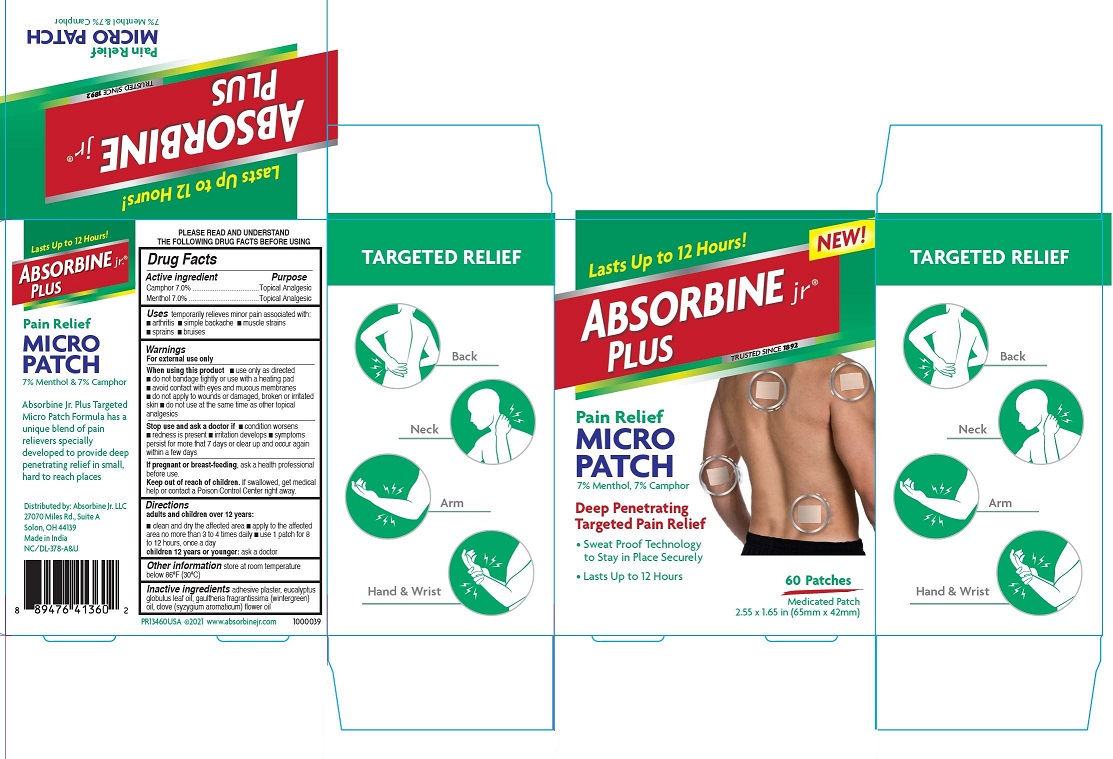
PRINCIPAL DISPLAY PANEL
Lasts Up to 12 Hours!
NEW!
Absorbine jr.® Plus
Pain Relief
MICRO PATCH
7% Menthol, 7% Camphor
Deep Penetrating
Targeted Pain Relief
• Sweat Proof Technology to Stay in Place Securely
• Lasts up to 12 Hours
60 Patches
Medicated Patch
2.55 × 1.65 in (65mm × 42mm)
◎ Back
◎ Neck
◎ Arm
◎ Hand & Wrist
Absorbine Jr. Plus Targeted Micro Patch Formula has a unique blend of pain relievers specially developed to provide deep penetrating relief in small, hard to reach places
Distributed by: Absorbine jr. LLC
27070 Miles Rd., Suite A,
Solon, OH 44139
Made in India
NC/DL-378-A&U
PR13460USA ©2021 www.absorbinejr.com 1000039
-
INGREDIENTS AND APPEARANCE
ABSORBINE JR. PLUS PAIN RELIEF MICRO
camphor, menthol patchProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69693-415 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Menthol, Unspecified Form (UNII: L7T10EIP3A) (Menthol, Unspecified Form - UNII:L7T10EIP3A) Menthol, Unspecified Form 7 mg in 100 mg Camphor (synthetic) (UNII: 5TJD82A1ET) (Camphor (Synthetic) - UNII:5TJD82A1ET) Camphor (synthetic) 7 mg in 100 mg Inactive Ingredients Ingredient Name Strength Eucalyptus Globulus Leaf (UNII: S546YLW6E6) Methyl Salicylate (UNII: LAV5U5022Y) Clove Oil (UNII: 578389D6D0) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69693-415-01 3 in 1 CARTON 01/01/2022 1 20 in 1 POUCH 1 38.22 mg in 1 PATCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 01/01/2022 Labeler - Clarion Brands, LLC (079742703)