Uses temporarily relieves minor pain associated with:
- arthritis
- simple backache
- muscle strains
- sprains
- bruises
When using this product
- use only as directed
- do not bandage tightly or use with a heating pad
- avoid contact with eyes and mucous membranes
- do not apply to wounds or damaged, broken or irritated skin
- do not use at the same time as other topical analgesics
Stop use and ask a doctor if
- condition worsens
- redness is present
- irritation develops
- symptoms persist for more that 7 days or clear up and occur again within a few days
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
adults and children over 12 years:
- clean and dry the affected area
- apply to the affected area no more than 3 to 4 times daily
- use 1 patch for 8 to 12 hours, once a day
children 12 years or younger: ask a doctor
Inactive ingredients adhesive plaster, eucalyptus globulus leaf oil, gaultheria fragrantissima (wintergreen) oil, clove (syzygum aromaticum) flower oil
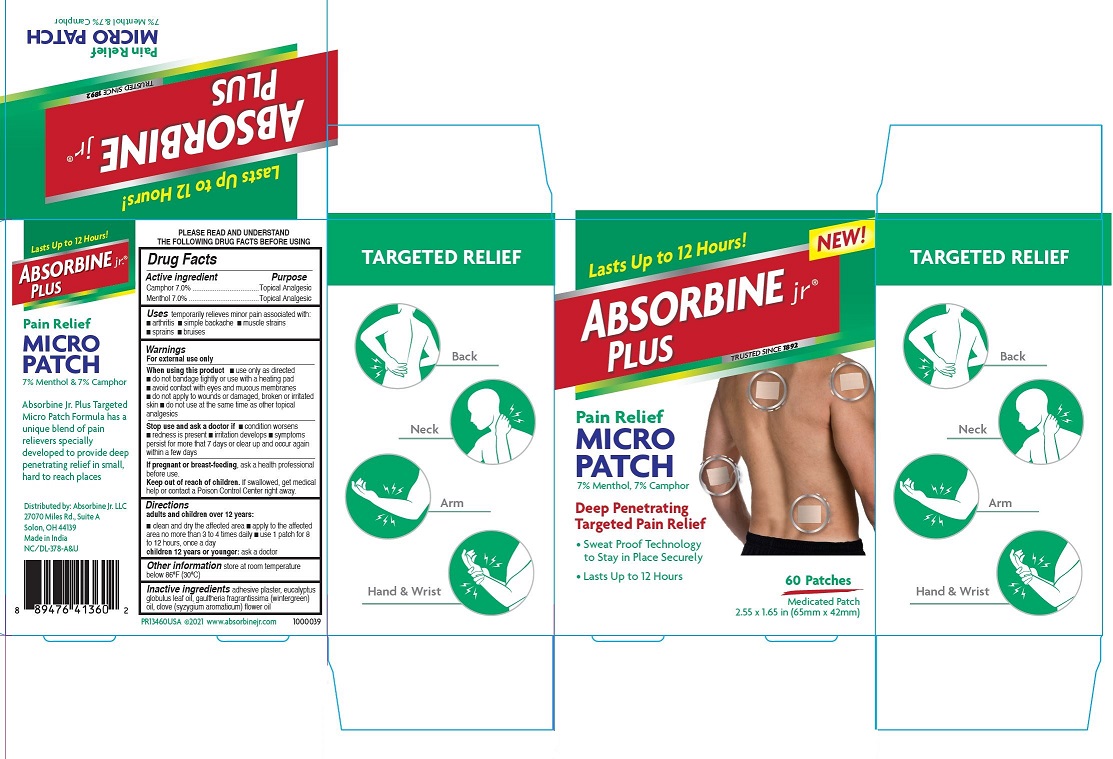
PRINCIPAL DISPLAY PANEL
Lasts Up to 12 Hours!
NEW!
Absorbine jr.® Plus
Pain Relief
MICRO PATCH
7% Menthol, 7% Camphor
Deep Penetrating
Targeted Pain Relief
• Sweat Proof Technology to Stay in Place Securely
• Lasts up to 12 Hours
60 Patches
Medicated Patch
2.55 × 1.65 in (65mm × 42mm)
◎ Back
◎ Neck
◎ Arm
◎ Hand & Wrist
Absorbine Jr. Plus Targeted Micro Patch Formula has a unique blend of pain relievers specially developed to provide deep penetrating relief in small, hard to reach places
Distributed by: Absorbine jr. LLC
27070 Miles Rd., Suite A,
Solon, OH 44139
Made in India
NC/DL-378-A&U
PR13460USA ©2021 www.absorbinejr.com 1000039