Label: ANTI-BACTERIAL HAND- ethyl alcohol gel
-
Contains inactivated NDC Code(s)
NDC Code(s): 50988-270-00, 50988-271-00, 50988-272-00, 50988-273-00, view more50988-274-00, 50988-275-00, 50988-276-00, 50988-277-00, 50988-278-00 - Packager: Jets, Sets, & Elephants Beauty Corp.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated December 6, 2011
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
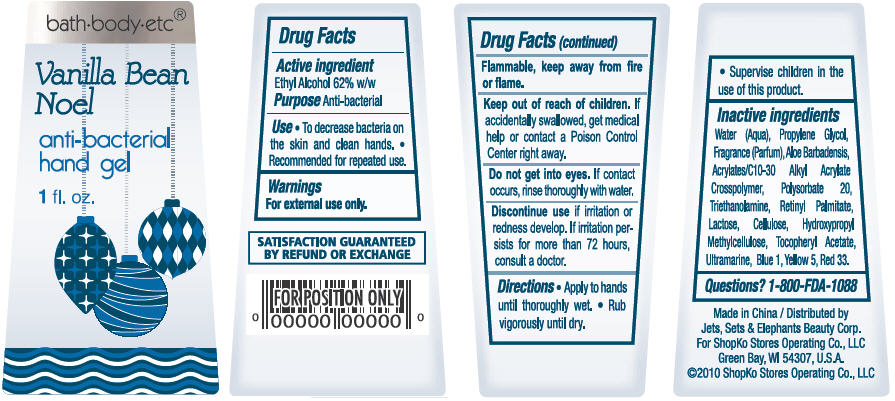
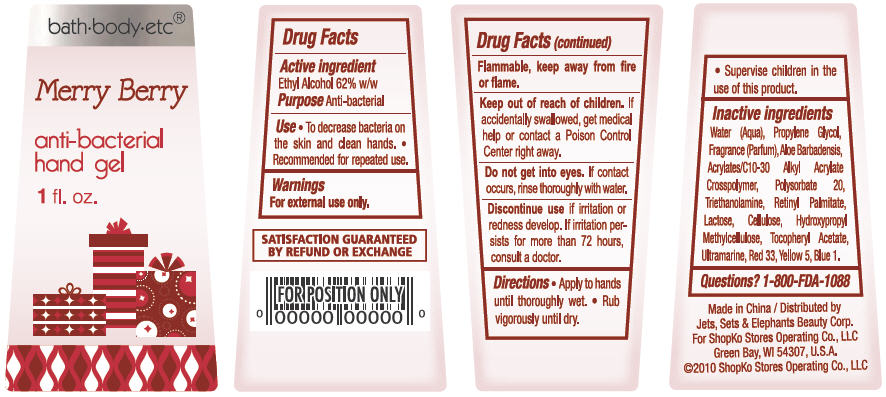
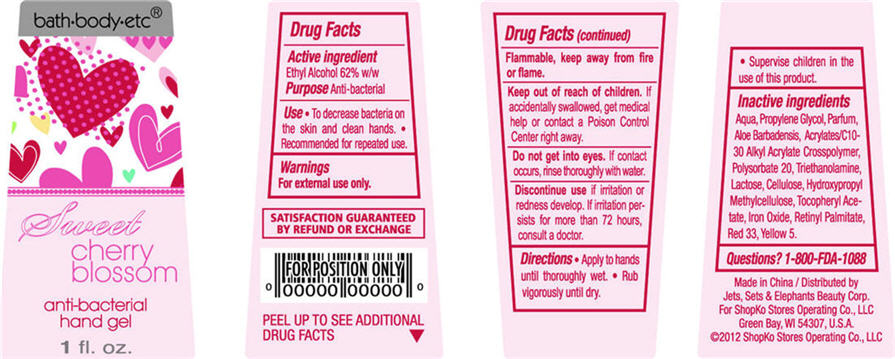
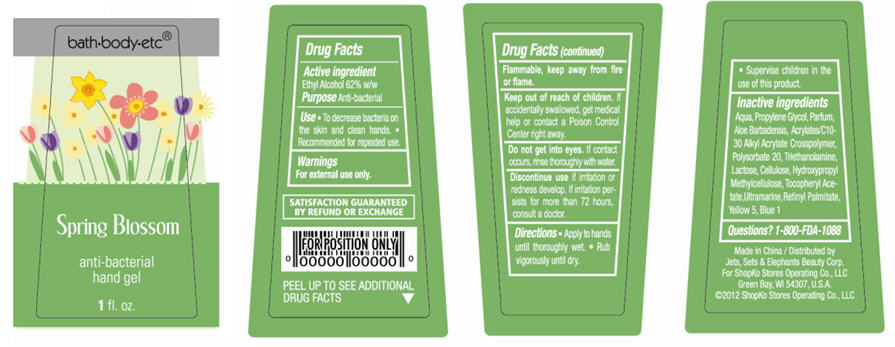
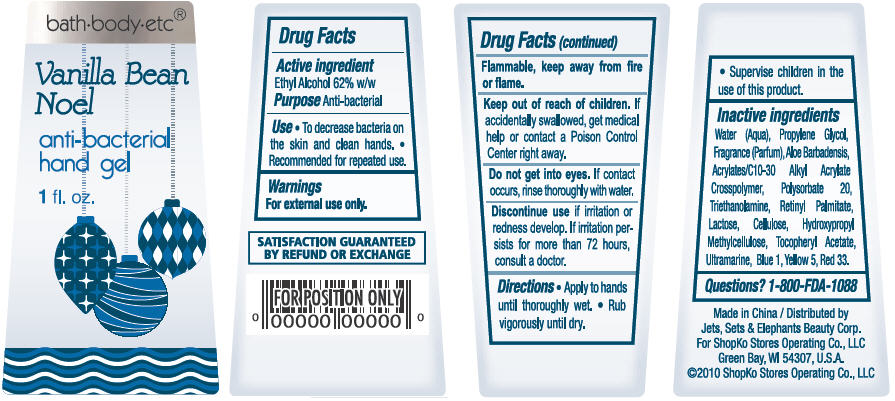
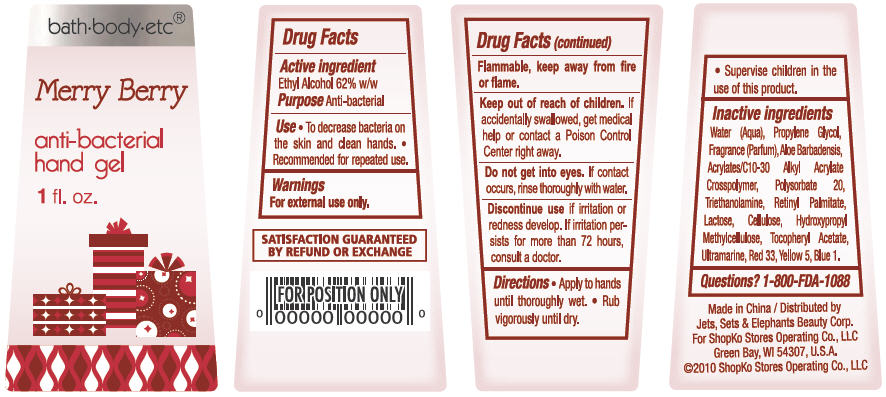
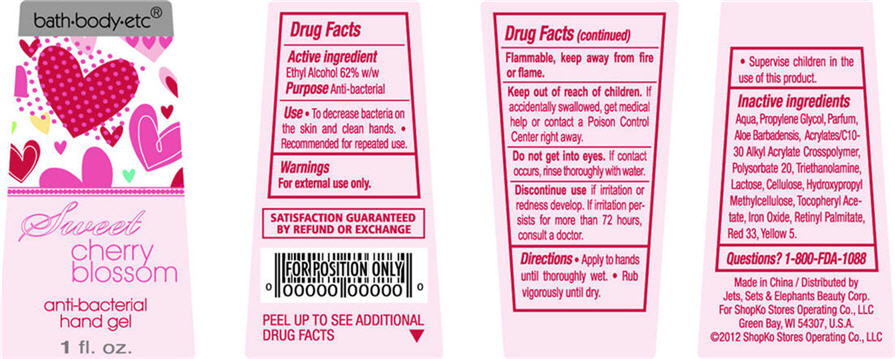
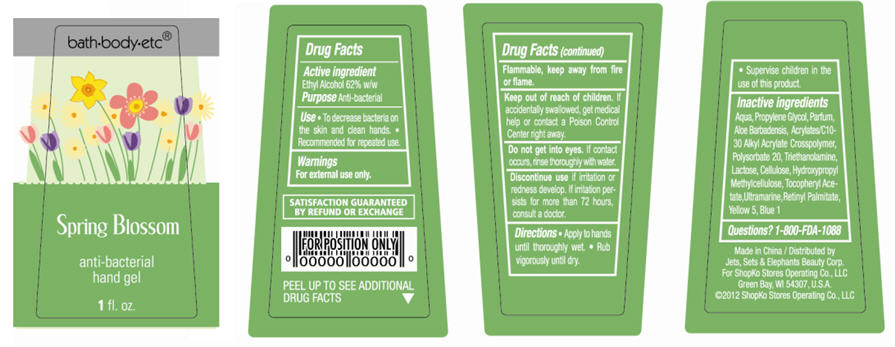
- ACTIVE INGREDIENT
- PURPOSE
- USE
- WARNINGS
- DIRECTIONS
-
Inactive Ingredients
Sweet Cherry Blossom
Water, Propylene Glycol, Parfum, Aloe Barbadensis, Acrylates/C10-30 Alkyl Acrylate Crosspolymer, Polysorbate 20, Triethanolamine, Lactose, Cellulose, Hydroxypropyl Methylcellulose, Tocopheryl Acetate, Iron Oxide, Retinyl Palmitate, Red 33, Yellow 5
Warm Vanilla Sugar
Water, Propylene Glycol, Parfum, Aloe Barbadensis, Acrylates/C10-30 Alkyl Acrylate Crosspolymer, Polysorbate 20, Triethanolamine, Lactose, Cellulose, Hydroxypropyl Methylcellulose, Tocopheryl Acetate, Iron Oxide, Retinyl Palmitate, Red 33, Yellow 5, Blue 1
Vanilla Bean Noel
Water, Propylene Glycol, Parfum, Aloe Barbadensis, Acrylates/C10-30 Alkyl Acrylate Crosspolymer, Polysorbate 20, Triethanolamine, Lactose, Cellulose, Hydroxypropyl Methylcellulose, Tocopheryl Acetate, Retinyl Palmitate, Ultramarine, Red 33, Yellow 5, Blue 1
Merry Berry
Water, Propylene Glycol, Parfum, Aloe Barbadensis, Acrylates/C10-30 Alkyl Acrylate Crosspolymer, Polysorbate 20, Triethanolamine, Lactose, Cellulose, Hydroxypropyl Methylcellulose, Tocopheryl Acetate, Retinyl Palmitate, Ultramarine, Red 33, Yellow 5, Blue 1
Peppermint Twist
Water, Propylene Glycol, Parfum, Aloe Barbadensis, Acrylates/C10-30 Alkyl Acrylate Crosspolymer, Polysorbate 20, Triethanolamine, Lactose, Cellulose, Hydroxypropyl Methylcellulose, Tocopheryl Acetate, Retinyl Palmitate, Ultramarine, Red 33, Yellow 5, Blue 1
Kiss and Tell
Water, Propylene Glycol, Parfum, Aloe Barbadensis, Acrylates/C10-30 Alkyl Acrylate Crosspolymer, Triethanolamine, Polysorbate 20, Lactose, Cellulose, Hydroxypropyl Methylcellulose, Tocopheryl Acetate, Iron Oxide, Retinyl Palmitate, Red 33, Yellow 5
Spring Fling
Water, Propylene Glycol, Parfum, Aloe Barbadensis, Acrylates/C10-30 Alkyl Acrylate Crosspolymer, Triethanolamine, Polysorbate 20, Lactose, Cellulose, Hydroxypropyl Methylcellulose, Tocopheryl Acetate, Chromium Hydroxide Green, Retinyl Palmitate, Yellow 5, Blue 1
Sweet Cherry Blossom
Water, Propylene Glycol, Parfum, Aloe Barbadensis, Acrylates/C10-30 Alkyl Acrylate Crosspolymer, Polysorbate 20, Triethanolamine, Lactose, Cellulose, Hydroxypropyl Methylcellulose, Tocopheryl Acetate, Iron Oxide, Retinyl Palmitate, Red 33, Yellow 5
Spring Blossom
Water, Propylene Glycol, Parfum, Aloe Barbadensis, Acrylates/C10-30 Alkyl Acrylate Crosspolymer, Polysorbate 20, Triethanolamine, Lactose, Cellulose, Hydroxypropyl Methylcellulose, Tocopheryl Acetate, Ultramarine, Retinyl Palmitate, Yellow 5, Blue 1
- QUESTIONS?
- SPL UNCLASSIFIED SECTION
- PACKAGE/LABEL PRINCIPAL DISPLAY PANEL - BOTTLE LABEL
- PACKAGE/LABEL PRINCIPAL DISPLAY PANEL - BOTTLE LABEL
- PACKAGE/LABEL PRINCIPAL DISPLAY PANEL - BOTTLE LABEL
- PACKAGE/LABEL PRINCIPAL DISPLAY PANEL - BOTTLE LABEL
- PACKAGE/LABEL PRINCIPAL DISPLAY PANEL - BOTTLE LABEL
- PACKAGE/LABEL PRINCIPAL DISPLAY PANEL - BOTTLE LABEL
- PACKAGE/LABEL PRINCIPAL DISPLAY PANEL - BOTTLE LABEL
- PACKAGE/LABEL PRINCIPAL DISPLAY PANEL - BOTTLE LABEL
- PACKAGE/LABEL PRINCIPAL DISPLAY PANEL - BOTTLE LABEL
-
INGREDIENTS AND APPEARANCE
ANTI-BACTERIAL HAND
ethyl alcohol gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:50988-270 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 18.335 mL in 29.574 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) ALOE VERA LEAF (UNII: ZY81Z83H0X) CARBOMER COPOLYMER TYPE B (ALLYL PENTAERYTHRITOL CROSSLINKED) (UNII: 1G56KV7BUJ) POLYSORBATE 20 (UNII: 7T1F30V5YH) TROLAMINE (UNII: 9O3K93S3TK) LACTOSE (UNII: J2B2A4N98G) .ALPHA.-TOCOPHEROL ACETATE, D- (UNII: A7E6112E4N) FERRIC OXIDE RED (UNII: 1K09F3G675) VITAMIN A PALMITATE (UNII: 1D1K0N0VVC) D&C RED NO. 33 (UNII: 9DBA0SBB0L) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:50988-270-00 29.574 mL in 1 BOTTLE, PLASTIC Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333A 04/11/2011 ANTI-BACTERIAL HAND
ethyl alcohol gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:50988-271 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 18.335 mL in 29.574 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) ALOE VERA LEAF (UNII: ZY81Z83H0X) CARBOMER COPOLYMER TYPE B (ALLYL PENTAERYTHRITOL CROSSLINKED) (UNII: 1G56KV7BUJ) POLYSORBATE 20 (UNII: 7T1F30V5YH) TROLAMINE (UNII: 9O3K93S3TK) LACTOSE (UNII: J2B2A4N98G) .ALPHA.-TOCOPHEROL ACETATE, D- (UNII: A7E6112E4N) FERRIC OXIDE RED (UNII: 1K09F3G675) VITAMIN A PALMITATE (UNII: 1D1K0N0VVC) D&C RED NO. 33 (UNII: 9DBA0SBB0L) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:50988-271-00 29.574 mL in 1 BOTTLE, PLASTIC Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333A 04/11/2011 ANTI-BACTERIAL HAND
ethyl alcohol gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:50988-272 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 18.335 mL in 29.574 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) ALOE VERA LEAF (UNII: ZY81Z83H0X) CARBOMER COPOLYMER TYPE B (ALLYL PENTAERYTHRITOL CROSSLINKED) (UNII: 1G56KV7BUJ) POLYSORBATE 20 (UNII: 7T1F30V5YH) TROLAMINE (UNII: 9O3K93S3TK) LACTOSE (UNII: J2B2A4N98G) .ALPHA.-TOCOPHEROL ACETATE, D- (UNII: A7E6112E4N) VITAMIN A PALMITATE (UNII: 1D1K0N0VVC) ULTRAMARINE BLUE (UNII: I39WR998BI) D&C RED NO. 33 (UNII: 9DBA0SBB0L) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:50988-272-00 29.574 mL in 1 BOTTLE, PLASTIC Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333A 08/10/2011 ANTI-BACTERIAL HAND
ethyl alcohol gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:50988-273 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 18.335 mL in 29.574 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) ALOE VERA LEAF (UNII: ZY81Z83H0X) CARBOMER COPOLYMER TYPE B (ALLYL PENTAERYTHRITOL CROSSLINKED) (UNII: 1G56KV7BUJ) POLYSORBATE 20 (UNII: 7T1F30V5YH) TROLAMINE (UNII: 9O3K93S3TK) LACTOSE (UNII: J2B2A4N98G) .ALPHA.-TOCOPHEROL ACETATE, D- (UNII: A7E6112E4N) VITAMIN A PALMITATE (UNII: 1D1K0N0VVC) ULTRAMARINE BLUE (UNII: I39WR998BI) D&C RED NO. 33 (UNII: 9DBA0SBB0L) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:50988-273-00 29.574 mL in 1 BOTTLE, PLASTIC Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333A 08/10/2011 ANTI-BACTERIAL HAND
ethyl alcohol gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:50988-274 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 18.335 mL in 29.574 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) ALOE VERA LEAF (UNII: ZY81Z83H0X) CARBOMER COPOLYMER TYPE B (ALLYL PENTAERYTHRITOL CROSSLINKED) (UNII: 1G56KV7BUJ) POLYSORBATE 20 (UNII: 7T1F30V5YH) TROLAMINE (UNII: 9O3K93S3TK) LACTOSE (UNII: J2B2A4N98G) .ALPHA.-TOCOPHEROL ACETATE, D- (UNII: A7E6112E4N) VITAMIN A PALMITATE (UNII: 1D1K0N0VVC) ULTRAMARINE BLUE (UNII: I39WR998BI) D&C RED NO. 33 (UNII: 9DBA0SBB0L) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:50988-274-00 29.574 mL in 1 BOTTLE, PLASTIC Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333A 08/10/2011 ANTI-BACTERIAL HAND
ethyl alcohol gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:50988-275 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 18.335 mL in 29.574 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) ALOE VERA LEAF (UNII: ZY81Z83H0X) CARBOMER COPOLYMER TYPE B (ALLYL PENTAERYTHRITOL CROSSLINKED) (UNII: 1G56KV7BUJ) POLYSORBATE 20 (UNII: 7T1F30V5YH) TROLAMINE (UNII: 9O3K93S3TK) LACTOSE (UNII: J2B2A4N98G) .ALPHA.-TOCOPHEROL ACETATE, D- (UNII: A7E6112E4N) FERRIC OXIDE RED (UNII: 1K09F3G675) VITAMIN A PALMITATE (UNII: 1D1K0N0VVC) D&C RED NO. 33 (UNII: 9DBA0SBB0L) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:50988-275-00 29.574 mL in 1 BOTTLE, PLASTIC Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333A 11/15/2011 ANTI-BACTERIAL HAND
ethyl alcohol gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:50988-276 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 18.335 mL in 29.574 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) ALOE VERA LEAF (UNII: ZY81Z83H0X) CARBOMER COPOLYMER TYPE B (ALLYL PENTAERYTHRITOL CROSSLINKED) (UNII: 1G56KV7BUJ) POLYSORBATE 20 (UNII: 7T1F30V5YH) TROLAMINE (UNII: 9O3K93S3TK) LACTOSE (UNII: J2B2A4N98G) .ALPHA.-TOCOPHEROL ACETATE, D- (UNII: A7E6112E4N) HYPROMELLOSE 2208 (100 MPA.S) (UNII: B1QE5P712K) VITAMIN A PALMITATE (UNII: 1D1K0N0VVC) CHROMIC OXIDE (UNII: X5Z09SU859) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:50988-276-00 29.574 mL in 1 BOTTLE, PLASTIC Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333A 11/15/2011 ANTI-BACTERIAL HAND
ethyl alcohol gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:50988-277 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 18.335 mL in 29.574 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) ALOE VERA LEAF (UNII: ZY81Z83H0X) CARBOMER COPOLYMER TYPE B (ALLYL PENTAERYTHRITOL CROSSLINKED) (UNII: 1G56KV7BUJ) POLYSORBATE 20 (UNII: 7T1F30V5YH) TROLAMINE (UNII: 9O3K93S3TK) LACTOSE (UNII: J2B2A4N98G) .ALPHA.-TOCOPHEROL ACETATE, D- (UNII: A7E6112E4N) FERRIC OXIDE RED (UNII: 1K09F3G675) VITAMIN A PALMITATE (UNII: 1D1K0N0VVC) D&C RED NO. 33 (UNII: 9DBA0SBB0L) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:50988-277-00 29.574 mL in 1 BOTTLE, PLASTIC Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333A 11/15/2011 ANTI-BACTERIAL HAND
ethyl alcohol gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:50988-278 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 18.335 mL in 29.574 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) ALOE VERA LEAF (UNII: ZY81Z83H0X) CARBOMER COPOLYMER TYPE B (ALLYL PENTAERYTHRITOL CROSSLINKED) (UNII: 1G56KV7BUJ) POLYSORBATE 20 (UNII: 7T1F30V5YH) TROLAMINE (UNII: 9O3K93S3TK) LACTOSE (UNII: J2B2A4N98G) .ALPHA.-TOCOPHEROL ACETATE, D- (UNII: A7E6112E4N) ULTRAMARINE BLUE (UNII: I39WR998BI) VITAMIN A PALMITATE (UNII: 1D1K0N0VVC) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:50988-278-00 29.574 mL in 1 BOTTLE, PLASTIC Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333A 11/15/2011 Labeler - Jets, Sets, & Elephants Beauty Corp. (243254039) Establishment Name Address ID/FEI Business Operations Gold Orient International Limited 679905914 MANUFACTURE(50988-270, 50988-271, 50988-272, 50988-273, 50988-274, 50988-275, 50988-276, 50988-277, 50988-278)