WARNINGS
For external use only. Flammable, keep away from fire or flame.
DIRECTIONS
- Apply to hands until thoroughly wet.
- Rub vigorously until dry.
- Supervise children in the use of this product.
Inactive Ingredients
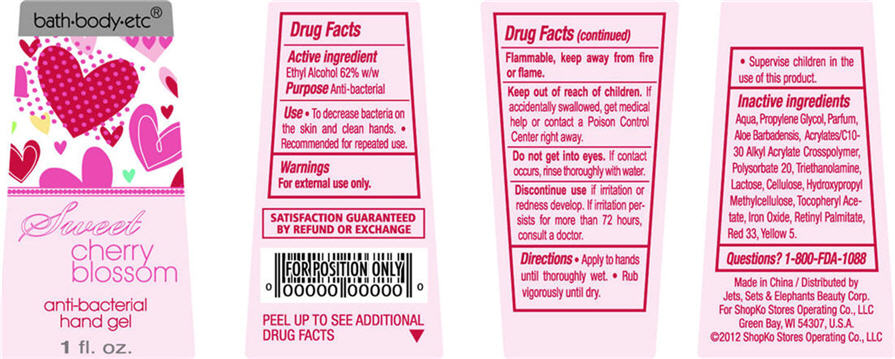
Sweet Cherry Blossom
Water, Propylene Glycol, Parfum, Aloe Barbadensis, Acrylates/C10-30 Alkyl Acrylate Crosspolymer, Polysorbate 20, Triethanolamine, Lactose, Cellulose, Hydroxypropyl Methylcellulose, Tocopheryl Acetate, Iron Oxide, Retinyl Palmitate, Red 33, Yellow 5
Warm Vanilla Sugar
Water, Propylene Glycol, Parfum, Aloe Barbadensis, Acrylates/C10-30 Alkyl Acrylate Crosspolymer, Polysorbate 20, Triethanolamine, Lactose, Cellulose, Hydroxypropyl Methylcellulose, Tocopheryl Acetate, Iron Oxide, Retinyl Palmitate, Red 33, Yellow 5, Blue 1
Vanilla Bean Noel
Water, Propylene Glycol, Parfum, Aloe Barbadensis, Acrylates/C10-30 Alkyl Acrylate Crosspolymer, Polysorbate 20, Triethanolamine, Lactose, Cellulose, Hydroxypropyl Methylcellulose, Tocopheryl Acetate, Retinyl Palmitate, Ultramarine, Red 33, Yellow 5, Blue 1
Merry Berry
Water, Propylene Glycol, Parfum, Aloe Barbadensis, Acrylates/C10-30 Alkyl Acrylate Crosspolymer, Polysorbate 20, Triethanolamine, Lactose, Cellulose, Hydroxypropyl Methylcellulose, Tocopheryl Acetate, Retinyl Palmitate, Ultramarine, Red 33, Yellow 5, Blue 1
Peppermint Twist
Water, Propylene Glycol, Parfum, Aloe Barbadensis, Acrylates/C10-30 Alkyl Acrylate Crosspolymer, Polysorbate 20, Triethanolamine, Lactose, Cellulose, Hydroxypropyl Methylcellulose, Tocopheryl Acetate, Retinyl Palmitate, Ultramarine, Red 33, Yellow 5, Blue 1
Kiss and Tell
Water, Propylene Glycol, Parfum, Aloe Barbadensis, Acrylates/C10-30 Alkyl Acrylate Crosspolymer, Triethanolamine, Polysorbate 20, Lactose, Cellulose, Hydroxypropyl Methylcellulose, Tocopheryl Acetate, Iron Oxide, Retinyl Palmitate, Red 33, Yellow 5
Spring Fling
Water, Propylene Glycol, Parfum, Aloe Barbadensis, Acrylates/C10-30 Alkyl Acrylate Crosspolymer, Triethanolamine, Polysorbate 20, Lactose, Cellulose, Hydroxypropyl Methylcellulose, Tocopheryl Acetate, Chromium Hydroxide Green, Retinyl Palmitate, Yellow 5, Blue 1
Sweet Cherry Blossom
Water, Propylene Glycol, Parfum, Aloe Barbadensis, Acrylates/C10-30 Alkyl Acrylate Crosspolymer, Polysorbate 20, Triethanolamine, Lactose, Cellulose, Hydroxypropyl Methylcellulose, Tocopheryl Acetate, Iron Oxide, Retinyl Palmitate, Red 33, Yellow 5
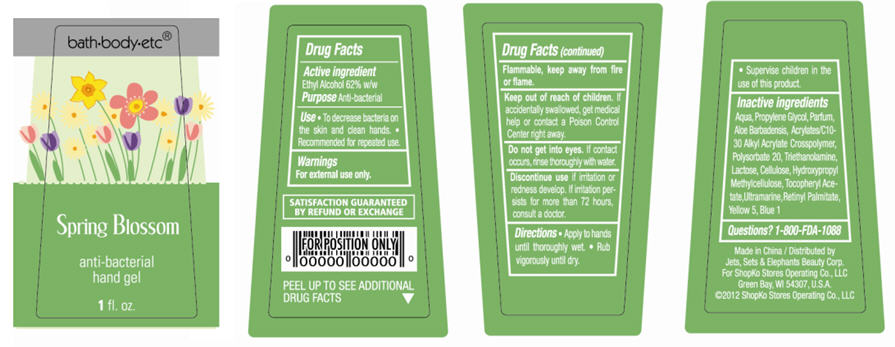
Spring Blossom
Water, Propylene Glycol, Parfum, Aloe Barbadensis, Acrylates/C10-30 Alkyl Acrylate Crosspolymer, Polysorbate 20, Triethanolamine, Lactose, Cellulose, Hydroxypropyl Methylcellulose, Tocopheryl Acetate, Ultramarine, Retinyl Palmitate, Yellow 5, Blue 1
Made in China
Distributed by Jets, Sets & Elephants Beauty Corp.
For ShopKo Stores Operating Co., LLC
Green Bay, WI 54307, U.S.A
©2010 ShopKo Stores Operating Co., LLC
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL - BOTTLE LABEL
bath-body-etc®
Sweet Cherry Blossom
anti-bacterial
hand gel
PARABEN FREE
contains aloe vera and
vitamins A & E
1 fl oz

Sweet Cherry Blossom Bottle Label
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL - BOTTLE LABEL
bath-body-etc®
Warm Vanilla Sugar
anti-bacterial
hand gel
PARABEN FREE
contains aloe vera and
vitamins A & E
1 fl oz

Warm Vanilla Sugar Bottle Label
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL - BOTTLE LABEL
bath-body-etc®
Vanilla Bean Noel
anti-bacterial
hand gel
1 fl oz
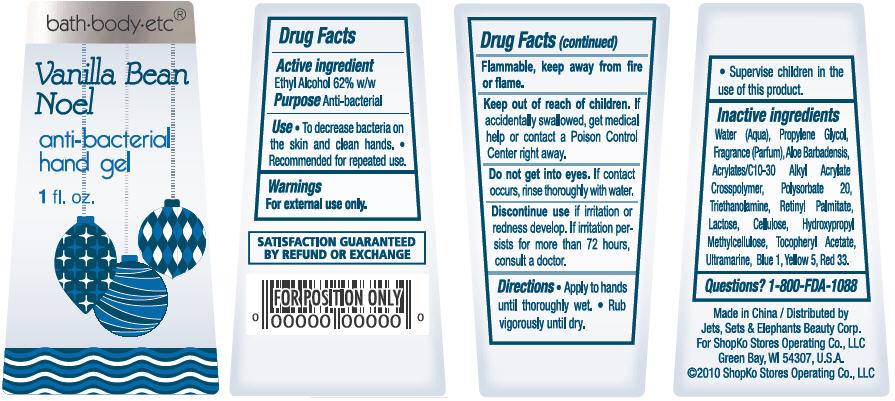
Vanilla Bean Noel Bottle Label
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL - BOTTLE LABEL
bath-body-etc®
Merry Berry
anti-bacterial
hand gel
1 fl oz
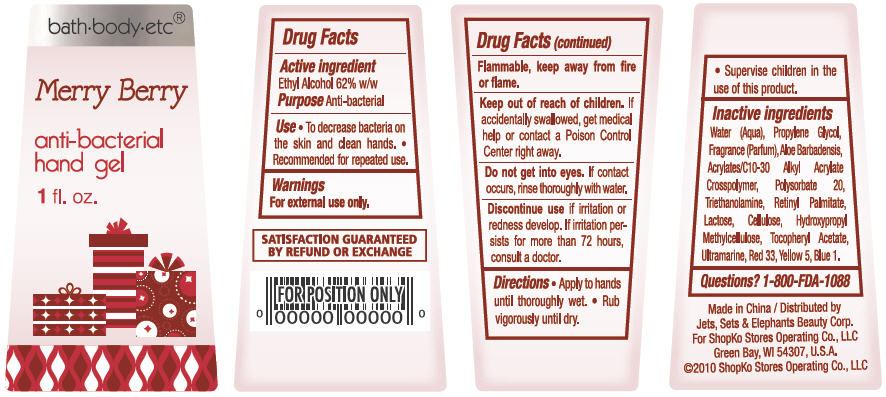
Merry Berry Bottle Label
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL - BOTTLE LABEL
bath-body-etc®
Peppermint Twist
anti-bacterial
hand gel
1 fl oz

Peppermint Twist Bottle Label
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL - BOTTLE LABEL
bath-body-etc®
Kiss and tell
anti-bacterial
hand gel
1 fl oz

Kiss and tell Bottle Label
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL - BOTTLE LABEL
bath-body-etc®
Spring fling
anti-bacterial
hand gel
1 fl oz

Spring fling Bottle Label