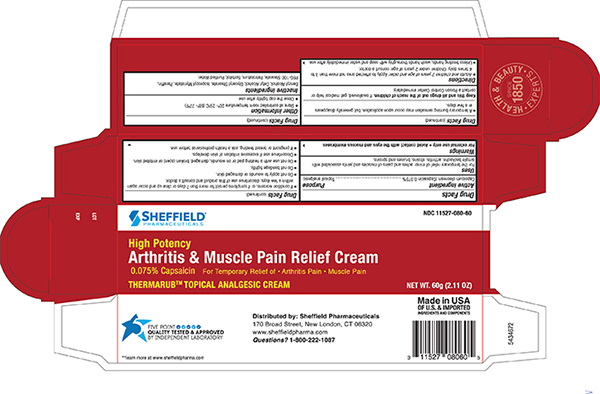
Label: SHEFFIELD ARTHRITIS AND MUSCLE PAIN RELIEF- capsicum oleoresin cream
- NDC Code(s): 11527-080-60
- Packager: Sheffield Pharmaceuticals LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 28, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active Ingredient
- Purpose
- Uses
-
Warnings
For external use only
Avoid contact with the eyes and mucous membranes- If condition worsens, or if symptoms persist for more than 7 days or clear up and occur again within a few days, discontinue use of this product and consult a doctor.
- Do not apply to wounds or damaged skin.
- Do not bandage tightly.
- Do not use with a heating pad or on wounds, damaged, broken (open) or irritated skin.
- Discontinue use if excessive irritation of skin develops.
- If pregnant or breast feeding, ask a health professional before use.
- A temporary burning sensation may occur upon application, but generally disappears in a few days
- Inactive ingredients
- Other Information
- Principal Display Panel – 60gm Carton Label
- Principal Display Panel – 60g Tube Label
-
INGREDIENTS AND APPEARANCE
SHEFFIELD ARTHRITIS AND MUSCLE PAIN RELIEF
capsicum oleoresin creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:11527-080 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CAPSICUM OLEORESIN (UNII: UW86K581WY) (CAPSICUM OLEORESIN - UNII:UW86K581WY) CAPSAICIN .075 g in 100 g Inactive Ingredients Ingredient Name Strength SORBITOL (UNII: 506T60A25R) WATER (UNII: 059QF0KO0R) ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) CETYL ALCOHOL (UNII: 936JST6JCN) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) PEG-100 STEARATE (UNII: YD01N1999R) BENZYL ALCOHOL (UNII: LKG8494WBH) PETROLATUM (UNII: 4T6H12BN9U) PARAFFIN (UNII: I9O0E3H2ZE) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:11527-080-60 1 in 1 CARTON 12/22/2020 1 60 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 12/22/2020 Labeler - Sheffield Pharmaceuticals LLC (151177797) Establishment Name Address ID/FEI Business Operations Sheffield Pharmaceuticals LLC 151177797 MANUFACTURE(11527-080) , analysis(11527-080)