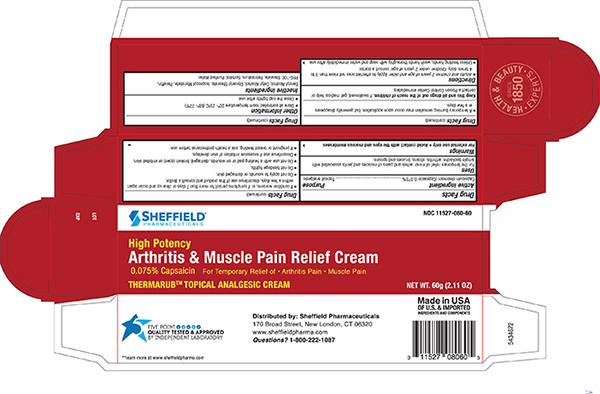
Uses
- For the temporary relief of minor aches and pains of muscles and joints associated with simple backache, arthritis,strains,bruises and sprains
Warnings
For external use only
Avoid contact with the eyes and mucous membranes
- If condition worsens, or if symptoms persist for more than 7 days or clear up and occur again within a few days, discontinue use of this product and consult a doctor.
- Do not apply to wounds or damaged skin.
- Do not bandage tightly.
- Do not use with a heating pad or on wounds, damaged, broken (open) or irritated skin.
- Discontinue use if excessive irritation of skin develops.
- If pregnant or breast feeding, ask a health professional before use.
- A temporary burning sensation may occur upon application, but generally disappears in a few days
Inactive ingredients
Benzyl Alcohol, Cetyl Alcohol, Glyceryl Sterate, Isopropyl Myristate, Paraffin, PEG-100 Stearate, Petrolatum, Purified Water , Sorbitol
Other Information
- Store at controlled room temperature 20°- 25°C (68° - 77°F)
- Close the cap tightly after use