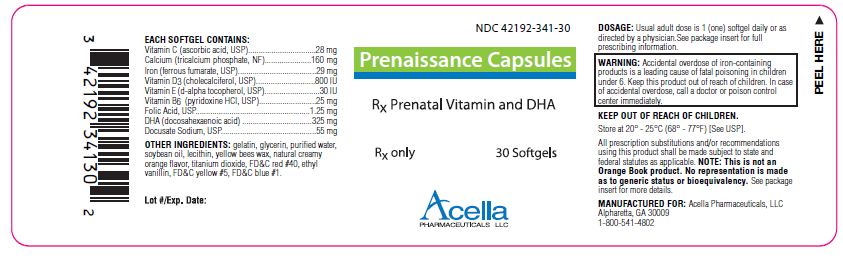
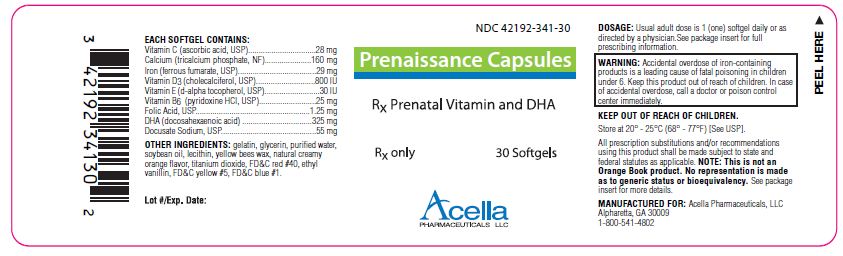
Label: PRENAISSANCE- calcium citrate, iron pentacarbonyl, cholecalciferol, .alpha.-tocopherol acetate, dl-, pyridoxine hydrochloride, folic acid, docusate sodium and doconexent capsule, liquid filled
- NDC Code(s): 42192-341-30
- Packager: Acella Pharmaceuticals, LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated January 16, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION:
Prenaissance Capsules are a prescription prenatal/postnatal multivitamin/multimineral softgel capsule with DHA. Each softgel is brown in color, opaque and imprinted with “341” on one side.
EACH SOFTGEL CONTAINS:
Vitamin C (ascorbic acid, USP) 28 mg Calcium (tricalcium phosphate, NF) 160 mg Iron (ferrous fumarate, USP) 29 mg Vitamin D3 (cholecalciferol, USP) 800 IU Vitamin E (d-alpha tocopherol, USP) 30 IU Vitamin B6 (pyridoxine HCl, USP) 25 mg Folic Acid, USP 1.25 mg DHA (docosahexaenoic acid) 325 mg Docusate Sodium, USP 55 mg - INDICATIONS:
- CONTRAINDICATIONS:
-
WARNING:
Ingestion of more than 3 grams of omega-3 fatty acids (such as DHA) per day has been shown to have potential antithrombotic effects, including an increased bleeding time and International Normalized Ratio (INR). Administration of omega-3 fatty acids should be avoided in patients taking anticoagulants and in those known to have an inherited or acquired predisposition to bleeding.
- BOXED WARNING (What is this?)
- PRECAUTIONS:
- ADVERSE REACTIONS:
- CAUTION:
- DOSAGE AND ADMINISTRATION:
- HOW SUPPLIED:
-
SPL UNCLASSIFIED SECTION
All prescription substitutions and/or recommendations using this product shall be made subject to state and federal statutes as applicable. Please note: this is not an Orange Book product and has not been subjected to FDA therapeutic equivalency or other equivalency testing. No representation is made as to generic status or bioequivalency. Each person recommending a prescription substitution using this product shall make such recommendations based on each such person’s professional opinion and knowledge, upon evaluating the active ingredients, excipients, inactive ingredients and chemical information provided herein.
MANUFACTURED FOR:
Acella Pharmaceuticals, LLC
Alpharetta, GA 30009
1-800-541-4802Rev. 0411
- PRINCIPAL DISPLAY PANEL - 30 Softgels
-
INGREDIENTS AND APPEARANCE
PRENAISSANCE
calcium citrate, iron pentacarbonyl, cholecalciferol, .alpha.-tocopherol acetate, dl-, pyridoxine hydrochloride, folic acid, docusate sodium and doconexent capsule, liquid filledProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:42192-341 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ASCORBIC ACID (UNII: PQ6CK8PD0R) (ASCORBIC ACID - UNII:PQ6CK8PD0R) ASCORBIC ACID 28 mg TRICALCIUM PHOSPHATE (UNII: K4C08XP666) (CALCIUM CATION - UNII:2M83C4R6ZB) CALCIUM CATION 160 mg FERROUS FUMARATE (UNII: R5L488RY0Q) (FERROUS CATION - UNII:GW89581OWR) FERROUS CATION 29 mg CHOLECALCIFEROL (UNII: 1C6V77QF41) (CHOLECALCIFEROL - UNII:1C6V77QF41) CHOLECALCIFEROL 800 [iU] .ALPHA.-TOCOPHEROL, D- (UNII: N9PR3490H9) (.ALPHA.-TOCOPHEROL, D- - UNII:N9PR3490H9) .ALPHA.-TOCOPHEROL, D- 30 [iU] PYRIDOXINE HYDROCHLORIDE (UNII: 68Y4CF58BV) (PYRIDOXINE - UNII:KV2JZ1BI6Z) PYRIDOXINE 25 mg FOLIC ACID (UNII: 935E97BOY8) (FOLIC ACID - UNII:935E97BOY8) FOLIC ACID 1.25 mg DOCONEXENT (UNII: ZAD9OKH9JC) (DOCONEXENT - UNII:ZAD9OKH9JC) DOCONEXENT 325 mg DOCUSATE SODIUM (UNII: F05Q2T2JA0) (DOCUSATE - UNII:M7P27195AG) DOCUSATE SODIUM 55 mg Inactive Ingredients Ingredient Name Strength GELATIN (UNII: 2G86QN327L) GLYCERIN (UNII: PDC6A3C0OX) WATER (UNII: 059QF0KO0R) SOYBEAN OIL (UNII: 241ATL177A) YELLOW WAX (UNII: 2ZA36H0S2V) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FD&C RED NO. 40 (UNII: WZB9127XOA) ETHYL VANILLIN (UNII: YC9ST449YJ) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) Product Characteristics Color BROWN Score no score Shape CAPSULE Size 25mm Flavor Imprint Code 341 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:42192-341-30 30 in 1 CAPSULE; Type 0: Not a Combination Product 06/13/2011 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date UNAPPROVED DRUG OTHER 06/13/2011 Labeler - Acella Pharmaceuticals, LLC (825380939) Registrant - Acella Pharmaceuticals, LLC (825380939) Establishment Name Address ID/FEI Business Operations Acella Pharmaceuticals, LLC 825380939 manufacture(42192-341)