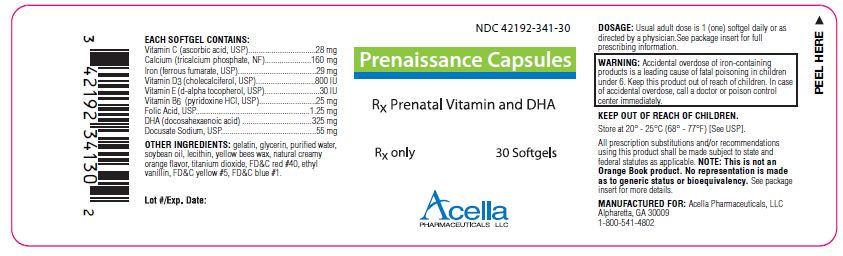
DESCRIPTION:
Prenaissance Capsules are a prescription prenatal/postnatal multivitamin/multimineral softgel capsule with DHA. Each softgel is brown in color, opaque and imprinted with “341” on one side.
EACH SOFTGEL CONTAINS:
| Vitamin C (ascorbic acid, USP) | 28 mg |
| Calcium (tricalcium phosphate, NF) | 160 mg |
| Iron (ferrous fumarate, USP) | 29 mg |
| Vitamin D3 (cholecalciferol, USP) | 800 IU |
| Vitamin E (d-alpha tocopherol, USP) | 30 IU |
| Vitamin B6 (pyridoxine HCl, USP) | 25 mg |
| Folic Acid, USP | 1.25 mg |
| DHA (docosahexaenoic acid) | 325 mg |
| Docusate Sodium, USP | 55 mg |
INDICATIONS:
Prenaissance Capsules are a multivitamin/multimineral nutritional supplement with DHA indicated for use in the dietary management of patients with nutritional deficiencies or are in need of nutritional supplementation.
CONTRAINDICATIONS:
This product is contraindicated in patients with a known hypersensitivity to any of the ingredients. Do not take this product if you are presently taking mineral oil, unless directed by a physician.
WARNING:
Ingestion of more than 3 grams of omega-3 fatty acids (such as DHA) per day has been shown to have potential antithrombotic effects, including an increased bleeding time and International Normalized Ratio (INR). Administration of omega-3 fatty acids should be avoided in patients taking anticoagulants and in those known to have an inherited or acquired predisposition to bleeding.
WARNING: Accidental overdose of iron-containing products is a leading cause of fatal poisoning in children under 6. Keep this product out of reach of children. In case of accidental overdose, call a doctor or poison control center immediately.
PRECAUTIONS:
Folic acid alone is improper therapy in the treatment of pernicious anemia and other megaloblastic anemias where vitamin B12 is deficient. Folic acid in doses above 1.0 mg daily may obscure pernicious anemia in that hematologic remission can occur while neurological manifestations progress.
ADVERSE REACTIONS:
Allergic sensitization has been reported following both oral and parenteral administration of folic acid.
CAUTION:
Exercise caution to ensure that the prescribed dosage of DHA does not exceed 1 gram (1000 mg) daily.
HOW SUPPLIED:
Prenaissance Capsules are supplied in child-resistant bottles of 30 softgels (NDC# 42192-341-30).
Store at 20° - 25°C (68° - 77°F) [See USP].
KEEP THIS AND ALL MEDICATIONS OUT OF THE REACH OF CHILDREN.
All prescription substitutions and/or recommendations using this product shall be made subject to state and federal statutes as applicable. Please note: this is not an Orange Book product and has not been subjected to FDA therapeutic equivalency or other equivalency testing. No representation is made as to generic status or bioequivalency. Each person recommending a prescription substitution using this product shall make such recommendations based on each such person’s professional opinion and knowledge, upon evaluating the active ingredients, excipients, inactive ingredients and chemical information provided herein.
MANUFACTURED FOR:
Acella Pharmaceuticals, LLC
Alpharetta, GA 30009
1-800-541-4802
Rev. 0411