Label: DUOPA- carbidopa and levodopa suspension
- NDC Code(s): 0074-3012-07
- Packager: AbbVie Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated May 7, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use DUOPA safely and effectively. See full prescribing information for DUOPA.
DUOPA® (carbidopa and levodopa) enteral suspension
Initial U.S. Approval: 1975
INDICATIONS AND USAGE
DUOPA is a combination of carbidopa (an aromatic amino acid decarboxylation inhibitor) and levodopa (an aromatic amino acid) indicated for the treatment of motor fluctuations in patients with advanced Parkinson’s disease (1)
DOSAGE AND ADMINISTRATION
- The maximum recommended daily dose of DUOPA is 2000 mg of levodopa (i.e., one cassette per day) administered over 16 hours (2.1)
- Prior to initiating DUOPA, convert patients from all forms of levodopa to oral immediate-release carbidopa-levodopa tablets (1:4 ratio) (2.2)
- Titrate total daily dose based on clinical response for the patient (2.2)
- Administer DUOPA into the jejunum through a percutaneous endoscopic gastrostomy with jejunal tube (PEG-J) with the CADD®-Legacy 1400 portable infusion pump (2.3)
DOSAGE FORMS AND STRENGTHS
Enteral Suspension: 4.63 mg carbidopa and 20 mg levodopa per mL (3)
CONTRAINDICATIONS
DUOPA is contraindicated in patients taking nonselective monoamine oxidase (MAO) inhibitors (4)
WARNINGS AND PRECAUTIONS
- Gastrointestinal procedure-related complications may result in serious outcomes, such as need for surgery or death (5.1)
- May cause falling asleep during activities of daily living (5.2)
- Monitor patients for orthostatic hypotension, especially after starting DUOPA or increasing the dose (5.3)
- Hallucinations/Psychosis/Confusion: May respond to dose reduction in levodopa (5.4)
- Impulse Control Disorders: Consider dose reductions or stopping DUOPA (5.5)
- Monitor patients for depression and suicidality (5.6)
- Avoid sudden discontinuation or rapid dose reduction to reduce the risk of withdrawal-emergent hyperpyrexia and confusion (5.7)
- May cause or exacerbate dyskinesia: Consider dose reduction (5.8)
- Monitor patients for signs and symptoms of peripheral neuropathy (5.9)
ADVERSE REACTIONS
Most common adverse reactions for DUOPA (DUOPA incidence at least 7% greater than oral carbidopa-levodopa incidence) were: complication of device insertion, nausea, depression, peripheral edema, hypertension, upper respiratory tract infection, oropharyngeal pain, atelectasis, and incision site erythema. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact AbbVie Inc. at 1-800-633-9110 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.DRUG INTERACTIONS
- Selective MAO-B inhibitors: May cause orthostatic hypotension (7.1)
- Antihypertensive drugs: May cause symptomatic postural hypotension. Dosage adjustment of the antihypertensive drug may be needed (7.2)
- Dopamine D2 receptor antagonists, isoniazid, iron salts, and high-protein diet may reduce the effectiveness of DUOPA (7.3, 7.4, 7.5)
USE IN SPECIFIC POPULATIONS
Pregnancy: Based on animal data, may cause fetal harm (8.1)
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 5/2025
- The maximum recommended daily dose of DUOPA is 2000 mg of levodopa (i.e., one cassette per day) administered over 16 hours (2.1)
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 DUOPA Daily Dose
2.2 Initiation and Titration Instructions
2.3 Administration Information
2.4 Discontinuation of DUOPA
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Gastrointestinal and Gastrointestinal Procedure-Related Risks
5.2 Falling Asleep During Activities of Daily Living and Somnolence
5.3 Orthostatic Hypotension
5.4 Hallucinations/Psychosis/Confusion
5.5 Impulse Control/Compulsive Behaviors
5.6 Depression and Suicidality
5.7 Withdrawal-Emergent Hyperpyrexia and Confusion
5.8 Dyskinesia
5.9 Neuropathy
5.10 Cardiovascular Ischemic Events
5.11 Laboratory Test Abnormalities
5.12 Glaucoma
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
7 DRUG INTERACTIONS
7.1 Monoamine Oxidase (MAO) Inhibitors
7.2 Antihypertensive Drugs
7.3 Dopamine D2 Receptor Antagonists and Isoniazid
7.4 Iron Salts
7.5 High-Protein Diet
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
16.2 Storage and Handling
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 DUOPA Daily Dose
DUOPA is administered over a 16-hour infusion period. The daily dose is determined by individualized patient titration and composed of:
- A Morning Dose
- A Continuous Dose
- Extra Doses
The maximum recommended daily dose of DUOPA is 2000 mg of the levodopa component (i.e., one cassette per day) administered over 16 hours. At the end of the daily 16-hour infusion, patients will disconnect the pump from the PEG-J and take their night-time dose of oral immediate-release carbidopa-levodopa tablets.
Treatment with DUOPA is initiated in 3 steps [see Dosage and Administration (2.2)]:
- Conversion of patients to oral immediate-release carbidopa-levodopa tablets in preparation for DUOPA treatment.
- Calculation and administration of the DUOPA starting dose (Morning Dose and Continuous Dose) for Day 1.
- Titration of the dose as needed based on individual clinical response and tolerability.
Extra Doses
DUOPA has an extra dose function that can be used to manage acute “Off” symptoms that are not controlled by the Morning Dose and the Continuous Dose administered over 16 hours. The extra dose function should be set at 1 mL (20 mg of levodopa) when starting DUOPA. If the amount of the extra dose needs to be adjusted, it is typically done in 0.2 mL increments. The extra dose frequency should be limited to one extra dose every 2 hours. Administration of frequent extra doses may cause or worsen dyskinesias.
Once no further adjustments are required to the DUOPA Morning Dose, Continuous Dose, or Extra Dose, this dosing regimen should be administered daily. Over time, additional changes may be necessary based on the patient’s clinical response and tolerability.
2.2 Initiation and Titration Instructions
Prepare for DUOPA Treatment
Prior to initiating DUOPA, convert patients from all other forms of levodopa to oral immediate-release carbidopa-levodopa tablets (1:4 ratio). Patients should remain on a stable dose of their concomitant medications taken for the treatment of Parkinson's disease before initiation of DUOPA infusion.
Healthcare providers should ensure patients take their oral Parkinson's disease medications the morning of the PEG-J procedure.
Determine the DUOPA Starting Dose for Day 1
The steps for determining the initial DUOPA daily dosing (Morning Dose and Continuous Dose) for Day 1 are outlined below.
Step 1: Calculate and administer the DUOPA Morning Dose for Day 1 a. Determine the total amount of levodopa (in milligrams) in the first dose of oral immediate-release carbidopa-levodopa that was taken by the patient on the previous day. b. Convert the oral levodopa dose from milligrams to milliliters by multiplying the oral dose by 0.8 and dividing by 20 mg/mL. This calculation will provide the Morning Dose of DUOPA in milliliters. c. Add 3 milliliters to the Morning Dose to fill (prime) the intestinal tube to obtain the Total Morning Dose. d. The Total Morning Dose is usually administered over 10 to 30 minutes. e. Program the pump to deliver the Total Morning Dose. Step 2: Calculate and administer the DUOPA Continuous Dose for Day 1 a. Determine the amount of oral immediate-release levodopa that the patient received from oral immediate-release carbidopa-levodopa doses throughout the previous day (16 waking hours), in milligrams. Do not include the doses of oral immediate-release carbidopa-levodopa taken at night when calculating the levodopa amount. b. Subtract the first oral levodopa dose in milligrams taken by the patient on the previous day (determined in Step 1 (a)) from the total oral levodopa dose in milligrams taken over 16 waking hours (determined in Step 2 (a)). Divide the result by 20 mg/mL. This is the dose of DUOPA administered as a Continuous Dose (in mL) over 16 hours. c. The hourly infusion rate (mL per hour) is obtained by dividing the Continuous Dose by 16 (hours). This value will be programmed into the pump as the continuous rate. d. If persistent or numerous “Off” periods occur during the 16-hour infusion, consider increasing the Continuous Dose or using the Extra Dose function. If dyskinesia or DUOPA-related adverse reactions occur, consider decreasing the Continuous Dose or stopping the infusion until the adverse reactions subside. DUOPA Titration
The daily dose of DUOPA can be titrated as needed, based on the patient’s individual clinical response and tolerability after Day 1 of DUOPA treatment and until a stable daily dose is maintained. Adjustments to concomitant Parkinson’s disease medications may be needed. In the controlled trial, the average number of titration days required to establish a stable Morning and Continuous Dose was 5 days. Additional dose adjustments may be necessary over time based on the patient level of activity and disease progression.
The recommendations for adjusting the DUOPA Morning and Continuous Doses are provided below.
Morning Dose Adjustment
If there was an inadequate clinical response within 1 hour of the Morning Dose on the preceding day, adjust the Morning Dose (excluding the 3 mL to fill the tube) as follows:
- If the Morning Dose on the preceding day was less than or equal to 6 mL, increase the Morning Dose by 1 mL.
- If the Morning Dose on the preceding day was greater than 6 mL, increase the Morning Dose by 2 mL.
If the patient experienced dyskinesias or DUOPA-related adverse reactions within 1 hour of the Morning Dose on the preceding day, decrease the Morning Dose by 1 mL.
Continuous Dose Adjustment
Consider increasing the Continuous Dose based on the number and volume of Extra Doses of DUOPA (i.e., total amount of levodopa component) that were needed for the previous day and the patient’s clinical response.
Consider decreasing the Continuous Dose if the patient experienced troublesome dyskinesia, or other troublesome DUOPA-related adverse reactions on the preceding day:
- For troublesome adverse reactions lasting for a period of one hour or more, decrease the Continuous Dose by 0.3 mL per hour.
- For troublesome adverse reactions lasting for two or more periods of one hour or more, decrease the Continuous Dose by 0.6 mL per hour.
2.3 Administration Information
- DUOPA should be used at room temperature. Take one DUOPA cassette out of the refrigerator and out of the carton 20 minutes prior to use; failure to use the product at room temperature may result in the patient not receiving the right amount of medication.
- DUOPA is delivered as a 16-hour infusion through either a naso-jejunal tube for short-term administration or through a PEG-J for long-term administration.
- The cassettes are for single-use only and should not be used for longer than 16 hours, even if some drug product remains.
- An opened cassette should not be re-used.
- The PEG-J should be disconnected from the pump at the end of the daily 16-hour administration period and flushed with room temperature potable water with a syringe.
Long-term administration of DUOPA requires placement of a PEG-J outer transabdominal tube and inner jejunal tube by percutaneous endoscopic gastrostomy. DUOPA is dispensed from medication cassette reservoirs that are specifically designed to be connected to the CADD®-Legacy 1400 pump.
Establishment of the transabdominal port should be performed by a gastroenterologist or other healthcare provider experienced in this procedure. See Table 1 for the recommended tubing sets for PEG-J administration.
For short-term, temporary administration of DUOPA prior to PEG-J tube placement, treatment may be initiated by a naso-jejunal tube with observation of the patient’s clinical response. See Table 2 for the recommended tubing sets for naso-jejunal administration.
Table 1. Recommended Tubing Sets for Long-Term PEG-J DUOPA Administration Product Name Manufacturer AbbVie PEG 15 and 20 Fr
AbbVie JAbbVie Inc.
AbbVie Inc.Table 2. Recommended Tubing Sets for Short-Term Naso-Jejunal DUOPA Administration Product Name Manufacturer AbbVie NJ AbbVie Inc. NJFT-10 Wilson-Cook Medical, Inc. Kangaroo™ Naso-Jejunal Feeding Tube Covidien Kangaroo™ Covidien 2.4 Discontinuation of DUOPA
Avoid sudden discontinuation or rapid dose reduction in patients taking DUOPA.
If patients need to discontinue DUOPA, the dose should be tapered or patients should be switched to oral immediate-release carbidopa-levodopa tablets [see Warnings and Precautions (5.7)].
When using a PEG-J tube, DUOPA can be discontinued by withdrawing the tube and letting the stoma heal. The removal of the tube should only be performed by a qualified healthcare provider.
- A Morning Dose
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
DUOPA is contraindicated in patients who are currently taking a nonselective monoamine oxidase (MAO) inhibitor (e.g., phenelzine and tranylcypromine) or have recently (within 2 weeks) taken a nonselective MAO inhibitor. Hypertension can occur if these drugs are used concurrently [see Drug Interactions (7.1 and 7.2)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Gastrointestinal and Gastrointestinal Procedure-Related Risks
Because DUOPA is administered using a PEG-J or naso-jejunal tube, gastrointestinal complications can occur.
These complications include abscess, bezoar, ileus, implant site erosion/ulcer, intestinal hemorrhage, intestinal ischemia, intestinal obstruction, intestinal perforation, intussusception, pancreatitis, peritonitis, pneumonia (including aspiration pneumonia), pneumoperitoneum, post-operative wound infection, and sepsis. These complications may result in serious outcomes, such as the need for surgery or death.
Instruct patients to notify their healthcare provider immediately if they experience abdominal pain, prolonged constipation, nausea, vomiting, fever, or melanotic stool [see Patient Counseling Information (17)].
5.2 Falling Asleep During Activities of Daily Living and Somnolence
Patients treated with levodopa, a component of DUOPA, have reported falling asleep while engaged in activities of daily living, including the operation of motor vehicles, which sometimes resulted in accidents. Although many of these patients reported somnolence while on levodopa, some perceived that they had no warning signs (sleep attack), such as excessive drowsiness, and believed that they were alert immediately prior to the event. Some of these events have been reported more than one year after initiation of treatment.
Falling asleep while engaged in activities of daily living usually occurs in patients experiencing preexisting somnolence, although patients may not give such a history. For this reason, prescribers should reassess patients for drowsiness or sleepiness in DUOPA-treated patients, especially since some of the events occur well after the start of treatment. Prescribers should be aware that patients may not acknowledge drowsiness or sleepiness until directly questioned about drowsiness or sleepiness during specific activities. Patients who have already experienced somnolence or an episode of sudden sleep onset should not participate in these activities while taking DUOPA.
Before initiating treatment with DUOPA, advise patients about the potential to develop drowsiness and specifically ask about factors that may increase the risk for somnolence with DUOPA such as the use of concomitant sedating medications or the presence of sleep disorders. Consider discontinuing DUOPA in patients who report significant daytime sleepiness or episodes of falling asleep during activities that require active participation (e.g., conversations, eating). If DUOPA is continued, they should be advised to avoid driving and other potentially dangerous activities that might result in harm if the patient becomes somnolent.
5.3 Orthostatic Hypotension
DUOPA-treated patients were more likely to experience a decline in orthostatic blood pressure than patients treated with oral immediate-release carbidopa-levodopa in the controlled clinical study. Orthostatic systolic hypotension (≥30 mm Hg decrease) occurred in 73% of DUOPA-treated patients compared to 68% of patients treated with oral immediate-release carbidopa-levodopa in the controlled clinical study. Orthostatic diastolic hypotension (≥20 mm Hg decrease) occurred in 70% of DUOPA-treated patients compared to 62% of patients treated with oral immediate-release carbidopa-levodopa. Inform patients about the risk for hypotension and syncope. Monitor patients for orthostatic hypotension, especially after starting DUOPA or increasing the dose.
5.4 Hallucinations/Psychosis/Confusion
There is an increased risk for hallucinations and psychosis in patients taking DUOPA. In the controlled clinical trial, hallucinations occurred in 5% of DUOPA-treated patients compared to 3% of patients treated with oral immediate-release carbidopa-levodopa. Confusion occurred in 8% of DUOPA-treated patients compared to 3% of patients treated with oral immediate-release carbidopa-levodopa, and psychotic disorder occurred in 5% of DUOPA-treated patients compared to 3% of patients treated with oral immediate-release carbidopa-levodopa.
Hallucinations associated with levodopa may present shortly after the initiation of therapy and may be responsive to dose reduction in levodopa. Confusion, insomnia, and excessive dreaming may accompany hallucinations. Abnormal thinking and behavior may present with one or more symptoms, including paranoid ideation, delusions, hallucinations, confusion, psychosis, disorientation, aggressive behavior, agitation, and delirium.
Because of the risk of exacerbating psychosis, patients with a major psychotic disorder should not be treated with DUOPA. In addition, medications that antagonize the effects of dopamine used to treat psychosis may exacerbate the symptoms of Parkinson’s disease and may decrease the effectiveness of DUOPA [see Drug Interactions (7.3)].
5.5 Impulse Control/Compulsive Behaviors
Patients may experience intense urges to gamble, increased sexual urges, intense urges to spend money, binge or compulsive eating, and/or other intense urges, and the inability to control these urges while taking one or more of the medications, including DUOPA, that increase central dopaminergic tone and that are generally used for the treatment of Parkinson’s disease. In some cases, although not all, these urges were reported to have stopped when the dose was reduced or the medication was discontinued.
Because patients may not recognize these behaviors as abnormal, it is important for prescribers to ask patients or their caregivers specifically about the development of new or increased gambling urges, sexual urges, uncontrolled spending, binge or compulsive eating, or other urges while being treated with DUOPA. Consider reducing the dose or discontinuing DUOPA if a patient develops such urges.
5.6 Depression and Suicidality
In the controlled clinical trial, 11% of DUOPA-treated patients developed depression compared to 3% of oral immediate-release carbidopa-levodopa-treated patients.
Monitor patients for the development of depression and concomitant suicidal tendencies.
5.7 Withdrawal-Emergent Hyperpyrexia and Confusion
A symptom complex that resembles neuroleptic malignant syndrome (characterized by elevated temperature, muscular rigidity, altered consciousness, and autonomic instability), with no other obvious etiology, has been reported in association with rapid dose reduction, withdrawal of, or changes in dopaminergic therapy. Avoid sudden discontinuation or rapid dose reduction in patients taking DUOPA. If DUOPA is discontinued, the dose should be tapered to reduce the risk of hyperpyrexia and confusion [see Dosage and Administration (2.4)].
5.8 Dyskinesia
DUOPA may cause or exacerbate dyskinesias. In the controlled clinical trial, dyskinesia occurred in 14% of DUOPA-treated patients compared to 12% of patients treated with oral immediate-release carbidopa-levodopa. The occurrence of dyskinesias may require a dosage reduction of DUOPA or other medications used to treat Parkinson’s disease.
5.9 Neuropathy
In clinical studies, 19 of 412 (5%) patients treated with DUOPA developed a generalized polyneuropathy. The onset of neuropathy could be determined in 13 of 19 patients. Most cases (12/19) were classified as subacute or chronic in onset. The neuropathy was most often characterized as sensory or sensorimotor. Electrodiagnostic testing performed in 16 patients was most often (15/16) consistent with an axonal polyneuropathy, and one patient was classified as having a demyelinating neuropathy. There was insufficient information to determine the potential role of vitamin deficiencies in the etiology of neuropathy associated with DUOPA.
Patients should have clinical assessments for the signs and symptoms of peripheral neuropathy before starting DUOPA. Monitor patients periodically for signs of neuropathy after starting DUOPA, especially in patients with pre-existing neuropathy and in patients taking medications or those who have medical conditions that are also associated with neuropathy.
5.10 Cardiovascular Ischemic Events
In clinical studies, myocardial infarction and arrhythmia were reported in patients taking carbidopa-levodopa. Ask patients about symptoms of ischemic heart disease and arrhythmia, especially those with a history of myocardial infarction or cardiac arrhythmias.
5.11 Laboratory Test Abnormalities
DUOPA may increase the risk for elevated (above the upper limit of normal for the reference range) blood urea nitrogen (BUN) and creatine phosphokinase (CPK). In the controlled clinical trial, the shift from a low or normal value at baseline to an increased BUN value was greater for DUOPA-treated patients (13%) than for patients treated with oral immediate-release carbidopa-levodopa (4%). The shift from a low or normal value at baseline to an increased CPK value was greater for DUOPA-treated patients (17%) than for patients treated with oral immediate-release carbidopa-levodopa (7%). The incidence of patients with a markedly increased BUN (≥10 mmol/L; ≥28 mg/dL) was greater for patients treated with DUOPA (11%) than that for patients treated with oral immediate-release carbidopa-levodopa (0%). The incidence of patients with an increased CPK (>3 times the upper limit of normal) was greater for patients treated with DUOPA (9%) than that for patients treated with oral immediate-release carbidopa-levodopa (0%).
Patients taking levodopa or carbidopa-levodopa may have increased levels of catecholamines and their metabolites in plasma and urine giving false positive results suggesting the diagnosis of pheochromocytoma in patients on levodopa and carbidopa-levodopa.
-
6 ADVERSE REACTIONS
The following serious adverse reactions are discussed below and elsewhere in labeling:
- Gastrointestinal and Gastrointestinal Procedure-Related Risks [see Warnings and Precautions (5.1)]
- Falling Asleep During Activities of Daily Living and Somnolence [see Warnings and Precautions (5.2)]
- Orthostatic Hypotension [see Warnings and Precautions (5.3)]
- Hallucinations/Psychosis/Confusion [see Warnings and Precautions (5.4)]
- Impulse Control/Compulsive Behaviors [see Warnings and Precautions (5.5)]
- Depression and Suicidality [see Warnings and Precautions (5.6)]
- Withdrawal-Emergent Hyperpyrexia and Confusion [see Warnings and Precautions (5.7)]
- Dyskinesia [see Warnings and Precautions (5.8)]
- Neuropathy [see Warnings and Precautions (5.9)]
- Cardiovascular Ischemic Events [see Warnings and Precautions (5.10)]
- Laboratory Test Abnormalities [see Warnings and Precautions (5.11)]
- Glaucoma [see Warnings and Precautions (5.12)]
6.1 Clinical Trials Experience
Because clinical studies are run under widely varying conditions, the incidence of adverse reactions observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
In clinical studies, 416 patients with advanced Parkinson’s disease received DUOPA. 338 patients were treated with DUOPA for more than 1 year, 233 patients were treated with DUOPA for more than 2 years, and 162 patients were treated with DUOPA for more than 3 years.
In a 12-week, active-controlled clinical trial (Study 1), a total of 71 patients with advanced Parkinson’s disease were enrolled and had a PEG-J procedure. Of these, 37 patients received DUOPA and 34 received oral immediate-release carbidopa-levodopa.
The most common adverse reactions for DUOPA (incidence at least 7% greater than oral immediate-release carbidopa-levodopa) were: complication of device insertion, nausea, depression, peripheral edema, hypertension, upper respiratory tract infection, oropharyngeal pain, atelectasis, and incision site erythema.
Table 3 lists the incidence of adverse reactions occurring in the DUOPA-treated group (requiring at least 2 patients in this group) in Study 1 when the incidence was numerically greater than that for oral immediate-release carbidopa-levodopa.
Table 3. Adverse Reactions in Study 1 for DUOPA in Patients with Advanced Parkinson’s disease Preferred Term DUOPA
(n = 37)
%Oral immediate-release carbidopa-levodopaa
(n = 34)
%Complication of device insertion 57 44 Nausea 30 21 Constipation 22 21 Incision site erythema 19 12 Dyskinesia 14 12 Depression 11 3 Post procedural discharge 11 9 Peripheral edema 8 0 Hypertension 8 0 Upper respiratory tract infection 8 0 Oropharyngeal pain 8 0 Atelectasis 8 0 Confusional state 8 3 Anxiety 8 3 Dizziness 8 6 Hiatal hernia 8 6 Postoperative ileus 5 0 Sleep disorder 5 0 Pyrexia 5 0 Excessive granulation tissue 5 0 Rash 5 0 Bacteriuria 5 0 White blood cells urine positive 5 0 Hallucination 5 3 Psychotic disorder 5 3 Diarrhea 5 3 Dyspepsia 5 3 aAll patients in the clinical trial regardless of treatment arm received a PEG-J. Procedure and Device- Related Adverse Reactions
The most common adverse reactions associated with complications due to naso-jejunal (NJ) insertion were: oropharyngeal pain, abdominal distention, abdominal pain, abdominal discomfort, pain, throat irritation, gastrointestinal injury, esophageal hemorrhage, anxiety, dysphagia, and vomiting.
The most common adverse reactions associated with complications due to PEG-J insertion were: abdominal pain, abdominal discomfort, abdominal distension, flatulence, or pneumoperitoneum.
Additional adverse reactions that were co-reported with complication of naso-jejunal and PEG-J insertion included upper abdominal pain, duodenal ulcer, duodenal ulcer hemorrhage, erosive duodenitis, erosive gastritis, gastrointestinal hemorrhage, intussusception, peritonitis, post-operative abscess, and small intestine ulcer.
- Gastrointestinal and Gastrointestinal Procedure-Related Risks [see Warnings and Precautions (5.1)]
-
7 DRUG INTERACTIONS
7.1 Monoamine Oxidase (MAO) Inhibitors
The use of nonselective MAO inhibitors with DUOPA is contraindicated [see Contraindications (4)]. Discontinue use of any nonselective MAO inhibitors at least two weeks prior to initiating DUOPA.
The use of selective MAO-B inhibitors (e.g., rasagiline and selegiline) with DUOPA may be associated with orthostatic hypotension. Monitor patients who are taking these drugs.
7.2 Antihypertensive Drugs
The concurrent use of DUOPA with antihypertensive medications can cause symptomatic postural hypotension. A dose reduction of the antihypertensive medication may be needed after starting or increasing the dose of DUOPA.
7.3 Dopamine D2 Receptor Antagonists and Isoniazid
Dopamine D2 receptor antagonists (e.g., phenothiazines, butyrophenones, risperidone, metoclopramide, papaverine) and isoniazid may reduce the effectiveness of levodopa. Monitor patients for worsening Parkinson’s symptoms.
7.4 Iron Salts
Iron salts or multi-vitamins containing iron salts can form chelates with levodopa, carbidopa, and can cause a reduction in the bioavailability of DUOPA. If iron salts or multi-vitamins containing iron salts are co-administered with DUOPA, monitor patients for worsening Parkinson’s symptoms.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no adequate data on the developmental risk associated with the use of DUOPA in pregnant women. In animal studies, carbidopa-levodopa has been shown to be developmentally toxic (including teratogenic effects) at clinically relevant doses (see Data).
The estimated background risk of major birth defects and miscarriage in the indicated population is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Data
Animal Data
When administered to pregnant rabbits throughout organogenesis, carbidopa-levodopa caused both visceral and skeletal malformations in fetuses at all doses and ratios of carbidopa-levodopa tested. No teratogenic effects were observed when carbidopa-levodopa was administered to pregnant mice throughout organogenesis. There was a decrease in the number of live pups delivered by rats receiving carbidopa-levodopa during organogenesis.
8.2 Lactation
Risk Summary
Levodopa has been detected in human milk after administration of carbidopa-levodopa. There are no data on the presence of carbidopa in human milk, the effects of levodopa or carbidopa on the breastfed infant, or the effects on milk production. However, inhibition of lactation may occur because levodopa decreases secretion of prolactin in humans. Carbidopa is excreted in rat milk.
The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for DUOPA and any potential adverse effects on the breastfed infant from DUOPA or from the underlying maternal condition.
8.5 Geriatric Use
In the controlled clinical trial, 49% of patients were 65 years and older, and 8% were 75 years and older. In patients 65 years and older, there was an increased risk for elevation of BUN and CPK (above the upper limit of the normal reference range for these laboratory analytes) during treatment with DUOPA compared to the risk for patients less than 65 years.
-
10 OVERDOSAGE
Management of acute overdosage with DUOPA is the same as management of acute overdosage with levodopa. Pyridoxine is not effective in reversing the actions of oral immediate-release carbidopa-levodopa.
In the event of an overdosage with DUOPA, the infusion should be stopped and the pump disconnected immediately. Administer intravenous fluids and maintain an adequate airway. Patients should receive electrocardiographic monitoring for arrhythmias and hypotension.
-
11 DESCRIPTION
DUOPA is a combination of carbidopa, an inhibitor of aromatic amino acid decarboxylation, and levodopa, an aromatic amino acid.
Carbidopa is a white, crystalline compound, slightly soluble in water, with a molecular weight of 244.2. It is designated chemically as (2S)-3-(3,4-dihydroxyphenyl)-2-hydrazino-2-methylpropanoic acid monohydrate. Its empirical formula is C10H14N2O4•H2O, and its structural formula is:
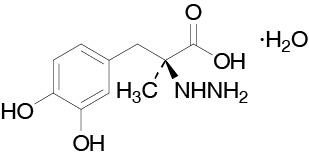
The content of carbidopa in DUOPA is expressed in terms of anhydrous carbidopa which has a molecular weight of 226.3. The 4.63 mg/mL of anhydrous carbidopa is equivalent to 5.0 mg/mL of carbidopa.
Levodopa is a white, crystalline compound, slightly soluble in water, with a molecular weight of 197.2. It is designated chemically as (2S)-2-Amino-3-(3,4-dihydroxyphenyl) propanoic acid. Its empirical formula is C9H11NO4, and its structural formula is:
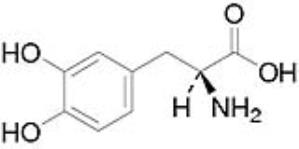
The inactive ingredients in DUOPA are carmellose sodium and purified water.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Carbidopa
When levodopa is administered orally, it is rapidly decarboxylated to dopamine in extracerebral tissues so that only a small portion of a given dose is transported unchanged to the central nervous system. Carbidopa inhibits the decarboxylation of peripheral levodopa, making more levodopa available for delivery to the brain.
Levodopa
Levodopa is the metabolic precursor of dopamine, does cross the blood-brain barrier, and presumably is converted to dopamine in the brain. This is thought to be the mechanism whereby levodopa treats the symptoms of Parkinson's disease.
12.2 Pharmacodynamics
Because its decarboxylase inhibiting activity is limited to extracerebral tissues, administration of carbidopa with levodopa makes more levodopa available to the brain. The addition of carbidopa to levodopa reduces the peripheral effects (e.g., nausea and vomiting) due to decarboxylation of levodopa; however, carbidopa does not decrease the adverse reactions due to the central effects of levodopa.
12.3 Pharmacokinetics
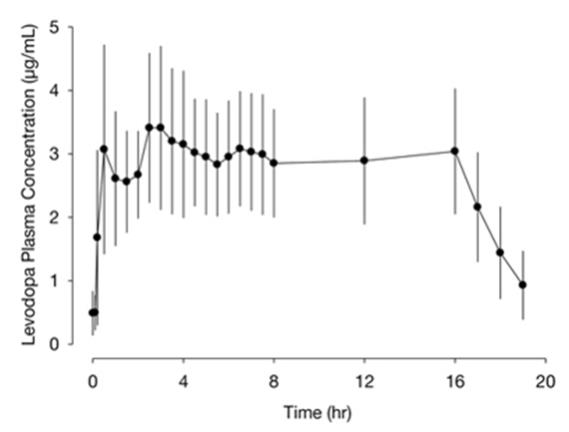
The pharmacokinetics of carbidopa and levodopa with 16-hour intrajejunal infusion of DUOPA was evaluated in 18 patients with advanced Parkinson's disease who had been on DUOPA therapy for 30 days or longer. Patients remained on their individualized DUOPA doses.
The plasma concentrations versus time profile for levodopa with DUOPA 16-hour intrajejunal infusion is presented in Figure 1.
Figure 1. Plasma Concentrations (mean ± standard deviation) versus Time Profile of Levodopa with DUOPA (levodopa, 1580 ± 403 mg; carbidopa, 366 ± 92 mg) 16-Hour Infusion
Absorption and Bioavailability
Following initiation of the 16-hour intrajejunal infusion of DUOPA, peak plasma levels of levodopa is reached at 2.5 hours. The absorption of levodopa may be decreased in patients on a high-protein diet because levodopa competes with certain amino acids for transport across the gut wall. The gastric emptying rate does not influence the absorption of DUOPA since it is administered by continuous intestinal infusion. In a cross-study population pharmacokinetic analysis, DUOPA had comparable bioavailability to the oral immediate-release carbidopa-levodopa (25/100 mg) tablets (over-encapsulated tablets). The estimated bioavailability for levodopa from DUOPA relative to oral immediate-release carbidopa-levodopa tablets was 97% (95% confidence interval; 95% to 98%).
In the controlled clinical trial, the intra-subject variability in carbidopa and levodopa plasma concentrations were lower for patients treated with DUOPA (N=33, 25% and 21%, respectively) than in patients treated with oral immediate-release carbidopa-levodopa (25/100 mg) tablets (N=28, 39% and 67%, respectively).
Distribution
Carbidopa is approximately 36% bound to plasma proteins. Levodopa is approximately 10-30% bound to plasma proteins.
Metabolism and Elimination
Carbidopa
Carbidopa is metabolized to two main metabolites (α-methyl-3-methoxy-4-hydroxyphenylpropionic acid and α-methyl-3,4-dihydroxyphenylpropionic acid). These 2 metabolites are primarily eliminated in the urine unchanged or as glucuronide conjugates. Unchanged carbidopa accounts for 30% of the total urinary excretion. The elimination half-life of carbidopa is approximately 2 hours.
Levodopa
Levodopa is mainly eliminated via metabolism by the aromatic amino acid decarboxylase (AAAD) and the catechol-O-methyl-transferase (COMT) enzymes. Other routes of metabolism are transamination and oxidation. The decarboxylation of levodopa to dopamine by AAAD is the major enzymatic pathway when no enzyme inhibitor is co-administered. O-methylation of levodopa by COMT forms 3-O-methyldopa. When administered with carbidopa, the elimination half-life of levodopa is approximately 1.5 hours (see Figure 1).
Drug Interaction Studies
COMT Inhibitors
Systemic exposure of levodopa is expected to increase in the presence of entacapone.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
In rat, oral administration of carbidopa-levodopa for two years resulted in no evidence of carcinogenicity. DUOPA contains hydrazine, a degradation product of carbidopa. In published studies, hydrazine has been demonstrated to be carcinogenic in multiple animal species. Increases in liver (adenoma, carcinoma) and lung (adenoma, adenocarcinoma) tumors have been reported with oral administration of hydrazine in mouse, rat, and hamster.
Mutagenesis
Carbidopa was positive in the in vitro Ames test, in the presence and absence of metabolic activation, and the in vitro mouse lymphoma tk assay in the absence of metabolic activation but was negative in the in vivo mouse micronucleus assay.
In published studies, hydrazine was reported to be positive in in vitro genotoxicity (Ames, chromosomal aberration in mammalian cells, and mouse lymphoma tk) assays and in the in vivo mouse micronucleus assay.
Impairment of Fertility
In reproduction studies, no effects on fertility were observed in rats receiving carbidopa-levodopa.
-
14 CLINICAL STUDIES
The efficacy of DUOPA was established in a randomized, double-blind, double-dummy, active-controlled, parallel group, 12-week study (Study 1) in patients with advanced Parkinson's disease who were levodopa-responsive and had persistent motor fluctuations while on treatment with oral immediate-release carbidopa-levodopa and other Parkinson's disease medications.
Patients were eligible for participation in the studies if they were experiencing 3 hours or more of “Off” time on their current Parkinson's disease drug treatment and they demonstrated a clear responsiveness to treatment with levodopa. Seventy-one (71) patients enrolled in the study and 66 patients completed the treatment (3 patients discontinued treatment because of adverse reactions, 1 patient for lack of effect, and 1 patient for non-compliance).
Patients enrolled in this study had a mean age of 64 years and disease duration of 11 years. Most patients (89%) were taking at least one concomitant medication for Parkinson’s disease (e.g., dopaminergic agonist, COMT-inhibitor, MAO-B inhibitor) in addition to oral immediate-release carbidopa-levodopa. Thirty nine percent of patients were taking two or more of such concomitant medications.
Patients were randomized to either DUOPA and placebo capsules or placebo suspension and oral immediate-release carbidopa-levodopa 25/100 mg capsules. Patients in both treatment arms had a PEG-J device placement. DUOPA or placebo-suspension was infused over 16 hours daily through a PEG-J tube via the CADD®-Legacy 1400 model ambulatory infusion pump. The mean daily levodopa dose was 1117 mg/day in the DUOPA group and 1351 mg/day in the oral immediate-release carbidopa-levodopa group.
The clinical outcome measure in Study 1 was the mean change from baseline to Week 12 in the total daily mean “Off” time, based on a Parkinson's disease diary. The "Off" time was normalized to a 16-hour awake period, based on a typical person's waking day and the daily infusion duration of 16 hours. The mean score decrease (i.e., improvement) in “Off” time from baseline to Week 12 for DUOPA was significantly greater (p=0.0015) than for oral immediate-release carbidopa-levodopa. Additionally, the mean score increase (i.e., improvement) in “On” time without troublesome dyskinesia from baseline to Week 12 was significantly greater (p=0.0059) for DUOPA than for oral immediate-release carbidopa-levodopa. The treatment difference (DUOPA – oral immediate release carbidopa-levodopa) for decrease in “Off” time was approximately 1.9 hours and the treatment difference for the increase in “On” time without troublesome dyskinesia was approximately 1.9 hours. Results of Study 1 are shown in Table 4.
Table 4. Change from Baseline to Week 12 in "Off" Time and in "On" Time Without Troublesome Dyskinesia in Patients with Advanced Parkinson’s Disease Treatment Group Baseline
(hours)LS Mean Change from Baseline at Week 12
(hours)"Off" time Oral immediate-release carbidopa-levodopa 6.9 -2.1 DUOPA 6.3 -4.0* "On" time without troublesome dyskinesia Oral immediate-release carbidopa-levodopa 8.0 2.2 DUOPA 8.7 4.1* LS Mean Change from Baseline based on Analysis of Covariance (ANCOVA).
*=Statistically Significant.Figure 2 shows results over time according to treatment for the efficacy variable (change from baseline in “Off” time) that served as the clinical outcome measure at the end of the trial at 12 weeks.
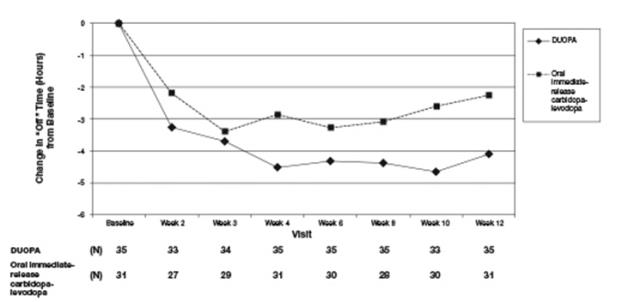
Figure 2. Change in “Off” Time Over 12 Weeks.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
Single-use cassettes containing 4.63 mg carbidopa (as 5 mg of the monohydrate) and 20 mg levodopa per mL of enteral suspension. Each cassette contains approximately 100 mL of suspension.
Carton of 7 DUOPA cassettes: NDC 0074-3012-07
16.2 Storage and Handling
Store in freezer at -20oC (-4oF). Thaw in refrigerator at 2oC to 8oC (36oF to 46oF) prior to dispensing. Cassettes should be protected from light and kept in the carton prior to use.
Thawing instructions for pharmacies
- Assign a 15 week “Use By” date based on the time the cartons are put into the refrigerator to thaw.
- Fully thaw DUOPA in the refrigerator prior to dispensing.
- In order to ensure controlled thawing of DUOPA, take the cartons containing the seven individual cassettes out of the transport box and separate the cartons from each other.
- Thawing may take up to 96 hours when the cartons are taken out of the transport box.
- Once the product has thawed, the individual cartons may be packed in a closer configuration within the refrigerator.
- Assign a 15 week “Use By” date based on the time the cartons are put into the refrigerator to thaw.
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide and Instructions for Use).
Administration Information
Ask patients if they have had any previous surgery in the upper part of their abdomen that may lead to difficulty in performing the gastrostomy or jejunostomy [see Dosage and Administration (2.3)].
Advise patients that foods that are high in protein may reduce the effectiveness of DUOPA [see Drug Interactions (7.5) and Clinical Pharmacology (12.3)].
Interruption of DUOPA Infusion
If the patient anticipates disconnecting the pump for a short period of time (less than 2 hours such as to swim, shower, or short medical procedure), no supplemental oral medication is needed, but the patient may be advised to take an extra-dose of DUOPA before disconnecting. Instruct the patient to stop the continuous rate, turn off the pump, clamp the cassette tube, disconnect the tubing, and replace the red cap on the cassette tube. The DUOPA cassette can remain attached to the pump until the tubing is reconnected. Refer the patient to the Patient Instructions for Use for additional information (i.e., changing the DUOPA Cassette: disconnecting Steps 1-5 and reconnecting Steps 10-16).
Advise the patient to contact their healthcare provider and to take oral carbidopa-levodopa until the patient is able to resume DUOPA infusion, if the patient will have prolonged interruption of therapy lasting more than 2 hours [see Dosage and Administration (2.4)].
Gastrointestinal and Gastrointestinal Procedure-Related Risks
Inform patients of the gastrointestinal procedure-related risks including abscess, bezoar, ileus, implant site erosion/ulcer, intestinal hemorrhage, intestinal ischemia, intestinal obstruction, intestinal perforation, intussusception, pancreatitis, peritonitis, pneumonia (including aspiration pneumonia), pneumoperitoneum, post-operative wound infection and sepsis. Advise patients of the symptoms of the above listed complications and instruct them to contact their healthcare provider if they experience any of these symptoms [see Warnings and Precautions (5.1)].
Falling Asleep during Activities of Daily Living and Somnolence
Alert patients to the potential sedating effects caused by DUOPA, including somnolence and the possibility of falling asleep while engaged in activities of daily living. Because somnolence is a common adverse reaction with potentially serious consequences, patients should not drive a car, operate machinery, or engage in other potentially dangerous activities until they have gained sufficient experience with DUOPA to gauge whether or not it affects their mental and/or motor performance adversely. Advise patients that if increased somnolence or episodes of falling asleep during activities of daily living (e.g., conversations, eating, driving a motor vehicle, etc.) are experienced at any time during treatment, they should not drive or participate in potentially dangerous activities until they have contacted their physician.
Advise patients of possible additive effects when patients are taking other sedating medications, alcohol, or other central nervous system depressants (e.g., benzodiazepines, antipsychotics, antidepressants, etc.) in combination with DUOPA or when taking a concomitant medication that increases plasma levels of levodopa [see Warnings and Precautions (5.2)].
Orthostatic Hypotension
Advise patients that they may experience syncope and may develop hypotension with or without symptoms such as dizziness, nausea, syncope, and sometimes sweating while taking DUOPA. Accordingly, caution patients against standing rapidly after sitting or lying down, especially if they have been doing so for prolonged periods and especially at the initiation of treatment with DUOPA [see Warnings and Precautions (5.3)].
Hallucinations/Psychosis/Confusion
Inform patients that they may experience hallucinations (unreal visions, sounds, or sensations) and other symptoms of psychosis can occur while taking DUOPA. Tell patients to report hallucinations, abnormal thinking, psychotic behavior or confusion to their healthcare provider promptly should they develop [see Warnings and Precautions (5.4)].
Impulse Control/Compulsive Behaviors
Advise patients that they may experience impulse control and/or compulsive behaviors while taking DUOPA. Advise patients to inform their physician or healthcare provider if they develop new or increased gambling urges, sexual urges, uncontrolled spending, binge or compulsive eating, or other urges while being treated with DUOPA [see Warnings and Precautions (5.5)].
Depression and Suicidality
Inform patients that they may develop depression or experience worsening of depression while taking DUOPA. Instruct patients to contact their healthcare provider if they experience depression, worsening of depression, or suicidal thoughts [see Warnings and Precautions (5.6)].
Withdrawal-Emergent Hyperpyrexia and Confusion
Advise patients to contact their healthcare provider before stopping DUOPA. Tell patients to inform their healthcare provider if they develop withdrawal symptoms such as fever, confusion, or severe muscle stiffness [see Warnings and Precautions (5.7)].
Dyskinesia
Inform patients that DUOPA may cause or exacerbate pre-existing dyskinesias [see Warnings and Precautions (5.8)].
Neuropathy
Inform patients that neuropathy may develop or they may experience worsening neuropathy on DUOPA, and to contact their healthcare provider if they develop any symptoms or features suggesting neuropathy [see Warnings and Precautions (5.9)].
Pregnancy
Advise patients to notify their healthcare provider if they become pregnant during treatment or plan to become pregnant during therapy [see Use in Specific Populations (8.1)].
Lactation
Advise patients to notify their healthcare provider if they are breastfeeding or plan to breastfeed [see Use in Specific Populations (8.2)].
Manufactured by AbbVie Inc., North Chicago, IL 60064, USA
or by HP Halden Pharma AS, Svinesundsveien 80, NO-1788 Halden, Norway
For AbbVie Inc.
North Chicago, IL 60064, USA© 2025 AbbVie. All rights reserved. DUOPA and its design are trademarks of AbbVie AB.
20087899R2 Revised: 5/2025 -
MEDICATION GUIDE
MEDICATION GUIDE
DUOPA (Do-oh-pa)
(carbidopa and levodopa) enteral suspension
Read this Medication Guide before you start using DUOPA and each time you get a refill. There may be new information. This information does not take the place of talking to your healthcare provider about your medical condition or treatment.
What is the most important information I should know about DUOPA?
DUOPA can cause serious side effects, including:
-
Stomach and intestine (gastrointestinal) problems and problems from the procedure you will need to have to receive DUOPA (gastrointestinal procedure-related problems).
Some of these problems may require surgery and may lead to death.
○ a blockage of your stomach or intestines (bezoar)
○ stopping movement through intestines (ileus)
○ drainage, redness, swelling, pain, feeling of warmth around the small hole in your stomach wall (stoma)
○ bleeding from stomach ulcers or your intestines
○ inflammation of your pancreas (pancreatitis)
○ infection in your lungs (pneumonia)
○ air or gas in your abdominal cavity
○ skin infection around the intestinal tube, pocket of infection (abscess), infection in your blood (sepsis) or abdominal cavity may occur, after surgery
○ stomach pain, nausea or vomiting
-
Tell your healthcare provider right away if you have any of the following symptoms of stomach and intestine problems and gastrointestinal procedure-related problems:
○ stomach (abdominal) pain
○ constipation that does not go away
○ nausea or vomiting
○ fever
○ blood in your stool or a dark tarry stool (melanotic stool)
You will need to have a procedure to make a small hole (called a “stoma”) in your stomach wall to place a gastro-jejunostomy tube (called a PEG-J tube) in an area of your small intestine called the jejunum. DUOPA is delivered directly to your small intestine through this tube. Your healthcare provider will talk to you about the stoma procedure. Before the stoma procedure, tell your healthcare provider if you have ever had a surgery or problems with your stomach.
Talk to your healthcare provider about what you need to do to care for your stoma. After the procedure, you and your healthcare provider will need to regularly check the stoma for any signs of infection.
If your PEG-J tube becomes kinked, knotted, or blocked this may cause you to have worsening of your Parkinson’s symptoms or recurring movement problems (motor fluctuations). Call your healthcare provider if your Parkinson’s symptoms get worse or you have slow movement while you are treated with DUOPA.
What is DUOPA?
DUOPA is a prescription medicine used for treatment of advanced Parkinson's disease. DUOPA contains 2 medicines, carbidopa and levodopa.
DUOPA should not be given to children (younger than 18 years).
Who should not use DUOPA?
Do not use DUOPA if you:
- take a medicine called a nonselective Monoamine Oxidase (MAO) Inhibitor (such as phenelzine or tranylcypromine) or have taken a nonselective MAO Inhibitor within the last 14 days.
Ask your healthcare provider or pharmacist if you are not sure if you take an MAO Inhibitor.
What should I tell my healthcare provider before using DUOPA?
Before you use DUOPA, tell your healthcare provider if you:
- have or have had stomach ulcers or stomach surgery
- have low blood pressure (hypotension) or if you feel dizzy or faint, especially when getting up from sitting or lying down
- have had problems with fainting (syncope)
- feel sleepy or have fallen asleep suddenly during the day
- have or have had depression (feelings of hopelessness or sadness) or any mental problems
- drink alcohol. Alcohol can increase the chance that DUOPA will make you feel sleepy or fall asleep when you should be awake
- have trouble controlling your muscles (dyskinesia)
- have nerve problems (peripheral neuropathy)
- have or have had heart problems, an abnormal heart rate or have had a heart attack in the past
- have or have had high blood pressure (hypertension)
- have eye problems that cause increased pressure in your eye (glaucoma)
- have a history of attacks of suddenly falling asleep and without warning
- have any other medical conditions
- are pregnant or planning to become pregnant. It is not known if DUOPA will harm your unborn baby
- are breastfeeding or plan to breastfeed. DUOPA can pass into your milk and may harm your baby. Talk to your healthcare provider about the best way to feed your baby if you take DUOPA
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, herbal supplements.
Using DUOPA with certain other medicines may affect each other and cause serious side effects.
Especially tell your healthcare provider if you take:
- medicines used to treat high blood pressure (hypertension)
- medicines used to treat depression called nonselective Monoamine Oxidase (MAO) Inhibitor (such as phenelzine or tranylcypromine) or have taken one within the last 14 days
- dopamine D2 receptor antagonists (antipsychotics or metoclopramide), and isoniazid
- iron or multivitamins with iron
Eating high protein foods may affect how DUOPA works. Tell your healthcare provider if you change your diet.
Ask your healthcare provider or pharmacist for a list of these medicines or foods if you are not sure.
Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist when you get a new medicine.
How should I use DUOPA?
- Use DUOPA exactly as your healthcare provider tells you to use it.
- Your healthcare provider should show you how to use DUOPA before you use it for the first time. Ask your healthcare provider or pharmacist if you have any questions.
- Your prescribed dose of DUOPA will be programmed into your pump by a healthcare provider and should only be changed by your healthcare provider or while you are with your healthcare provider.
-
Do not stop using DUOPA or change your dose unless you are told to do so by your healthcare provider. Tell your healthcare provider if you develop withdrawal symptoms such as fever, confusion, or severe muscle stiffness.
- Keep a supply of oral carbidopa-levodopa immediate release (IR) tablets with you in case you are unable to give your DUOPA infusion.
- DUOPA is given continuously over 16 hours through a tube that is put into your stomach called a PEG-J. A small pump (CADD-Legacy 1400) is used to move DUOPA from the medication cassette through your PEG-J tube.
- Your DUOPA dose has three parts:
○ a morning dose
○ a continuous dose
○ extra doses
- DUOPA can also be given for a short time (short-term) through a tube put into your nose called a naso-jejunal (NJ) tube.
- The CADD-Legacy 1400 portable infusion pump should be used to give DUOPA through your PEG-J tube. See the Instructions for Use that comes with your CADD-Legacy 1400 portable infusion pump for complete instructions on how to use the pump.
- DUOPA comes in a small plastic container (cassette) that you connect to the pump to get your medicine.
○ Each cassette can only be used 1 time. An opened cassette should not be reused.
○ The cassette should not be used for longer than 16 hours.
○ The cassette should be thrown away at the end of the infusion, even if there is some medicine still in the cassette.
- Disconnect the pump from your PEG-J tube after the 16 hour dosing time is finished. Use a syringe filled with room temperature water to flush your PEG-J tube. See the “Instructions for Use” for more information about how to flush your PEG-J tube with a syringe.
- After your daily DUOPA infusion, you should take your usual night-time dose of oral carbidopa-levodopa tablets as prescribed.
- If you stop your DUOPA infusion for more than 2 hours during your 16 hour dosing time for any reason, call your healthcare provider and take oral carbidopa-levodopa as prescribed until you are able to restart your DUOPA infusion.
- If you stop your DUOPA infusion for less than 2 hours, you do not need to take oral carbidopa-levodopa, but your healthcare provider may tell you to take an extra dose of DUOPA.
What should I avoid while using DUOPA?
- Do not drive, operate machinery, or do other activities until you know how DUOPA affects you. Sleepiness and falling asleep suddenly caused by DUOPA can happen as late as 1 year after you start your treatment.
What are the possible side effects of DUOPA?
DUOPA may cause serious side effects, including:
-
See “What is the most important information I should know about DUOPA?”
-
Falling asleep during normal daily activities. DUOPA may cause you to fall asleep while you are doing daily activities such as driving, talking with other people, or eating.
○ You could fall asleep without any warning.
○ Some people using DUOPA have had car accidents because they fell asleep while driving.
Do not drive or operate machinery until you are sure how DUOPA affects you.
Tell your healthcare provider if you take other medicines that can make you sleepy such as sleep medicines, antidepressants, or antipsychotics.
-
Low blood pressure when you sit or stand up quickly. After you have been sitting or lying down, stand up slowly until you know how DUOPA affects you. This may help reduce the following symptoms while you are using DUOPA:
○ dizziness
○ nausea
○ sweating
○ fainting
-
Seeing things that are not there, hearing sounds or feeling sensations that are not real (hallucinations). Hallucinations can happen in people who use DUOPA. Tell your healthcare provider if you have hallucinations.
-
Unusual urges. Some people taking certain medicines to treat Parkinson’s disease, including DUOPA, have reported problems, such as gambling, compulsive eating, compulsive shopping, and increased sex drive.
If you or your family members notice that you are having unusual urges or behaviors, talk to your healthcare provider.
-
Depression and suicide. DUOPA can cause depression or make your depression worse. Pay close attention to sudden changes in your mood, behavior, thoughts, or feelings. Call your healthcare provider right away if you feel depressed or have thoughts of suicide.
-
Uncontrolled sudden movements (dyskinesia). If you have new dyskinesia, or your dyskinesia gets worse, tell your healthcare provider. This may be a sign that your dose of DUOPA or other medicines to control your Parkinson’s disease may need to be adjusted.
-
Progressive weakness or numbness or loss of sensation in the fingers or feet (neuropathy).
-
Heart attack or other heart problems. Tell your healthcare provider if you have experienced increased blood pressure, a fast or irregular heartbeat or chest pain.
-
Abnormal blood tests. DUOPA may cause changes in certain blood tests, especially certain hormone and kidney function blood tests.
-
Worsening of the increased pressure in your eyes (glaucoma). The pressure in your eyes should be checked after starting DUOPA.
-
The most common side effects of DUOPA include:
○ swelling of legs and feet
○ nausea
○ high blood pressure (hypertension)
○ depression
○ mouth and throat pain
Call your healthcare provider or get medical care right away if you have any of the above symptoms. Your healthcare provider will tell you if you should stop treatment with DUOPA and if needed, tell you how to discontinue DUOPA.
Tell your healthcare provider if you have any side effect that bothers you or does not go away.
These are not all of the possible side effects of DUOPA. For more information, ask your healthcare provider or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store DUOPA?
- Store DUOPA in the refrigerator between 36ºF to 46ºF (2ºC to 8ºC). Do not freeze.
- Use at room temperature. Take one DUOPA cassette out of the carton and out of the refrigerator 20 minutes prior to use. Use the product at room temperature or you may not get the right amount of medication.
- Protect the cassette from light and keep it in the carton before using.
- Use DUOPA before the expiration date printed on the cassette.
Keep DUOPA and all medicines out of the reach of children.
General information about the safe and effective use of DUOPA.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use DUOPA for a condition for which it was not prescribed. Do not give DUOPA to other people, even if they have the same symptoms that you have. It may harm them.
This Medication Guide summarizes the most important information about DUOPA. If you would like more information, talk with your healthcare provider. You can ask your healthcare provider or pharmacist for information about DUOPA that was written for healthcare professionals.
For more information go to www.DUOPA.com or call 1-844-386-4968.
What are the ingredients in DUOPA?
Active ingredients: carbidopa and levodopa
Inactive ingredients: carmellose sodium and purified water
This Medication Guide has been approved by the U.S. Food and Drug Administration.
Manufactured by AbbVie Inc., North Chicago, IL 60064, USA
or by HP Halden Pharma AS, Svinesundsveien 80, NO-1788 Halden, Norway
For AbbVie Inc.
North Chicago, IL 60064, USA© 2025 AbbVie. All rights reserved. DUOPA and its design are trademarks of AbbVie AB.
20087899R2 Revised: 5/2025 -
Stomach and intestine (gastrointestinal) problems and problems from the procedure you will need to have to receive DUOPA (gastrointestinal procedure-related problems).
-
INSTRUCTIONS FOR USE
INSTRUCTIONS FOR USE
DUOPA
(carbidopa and levodopa) enteral suspension
These instructions are for use along with any other instructions your healthcare provider gives you.
Please read the Medication Guide before you start using DUOPA and each time you get a refill.
For questions or problems, call DUOPA support toll free at 1-844-386-4968.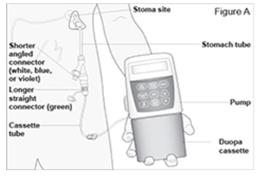
The CADD-Legacy® 1400 pump is used for delivery of DUOPA through a tube into your stomach attached to the longer straight (green) connector. Enteral nutrition should only be given by the shorter angled connector (white, blue, or violet) (see Figure A and Table. Connector Colors). Table. Connector Colors. Y-Connector
SizeDesign Gastric
(“g”) Port
ColorIntestinal
(“i”) Port
Color15 FR Original White New Blue Green 20 FR Original White New Violet Note: The original design of the Y-Connector is represented in the Figures throughout this Instructions for Use.
This Instructions for Use provides information for the CADD-Legacy® model 1400 pump only. There are other CADD-Legacy® pump models available. Read the label on the back of the pump to make sure it is a model 1400 pump.
Your healthcare provider prescribed DUOPA for you. Your healthcare provider programs your prescription into the CADD-Legacy® 1400 pump. The CADD-Legacy® 1400 pump is approved for use with DUOPA. DUOPA is provided as medication inside cassettes that connect to the CADD-Legacy® 1400 pump.The pump delivers DUOPA in 3 ways:
- Continuous Rate: Steady delivery of DUOPA delivered throughout the day while pump is on
- Morning Dose: A large dose of DUOPA given each morning
- Extra Dose: A small dose of DUOPA given as needed during the day
You will need the following items to complete these steps:
- Pump
- DUOPA cassette
- Coin, like a quarter
- Carrying bag
- Syringe
- Syringe connector
- Room temperature water
CADD-Legacy®-1400 Pump 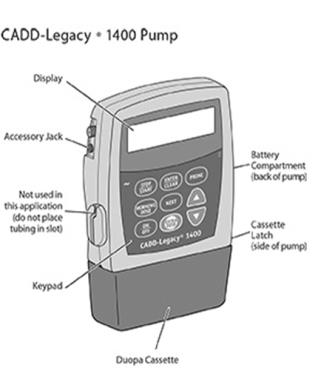

Display
The display shows programming information and messages. The main screen, which the pump displays most of the time, shows the following: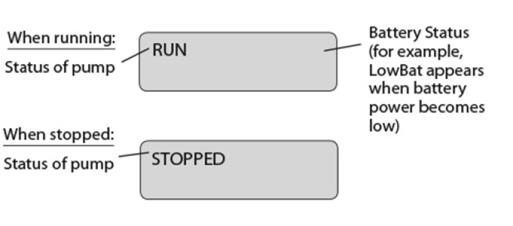
DUOPA Cassette
The single-use DUOPA cassette is for use with the CADD-Legacy® 1400 pump.
Battery Compartment
Two AA batteries fit into the battery compartment.
Cassette Latch
The cassette latch secures the DUOPA cassette to the pump.WARNINGS and CAUTIONS
Failure to follow the Warnings and Cautions below could cause return of your symptoms, damage to the pump, serious injury, or may lead to death in rare cases.
WARNINGS
- Only use the pump in a manner described in this Instructions for Use, after you have received training by your healthcare provider.
- To avoid explosion hazard, do not use the pump near flammable explosive gases.
- Only use extension sets that are approved for use with DUOPA (See the Full Prescribing Information for DUOPA), pay attention to all warnings and cautions associated with their use.
- Always have new batteries available for replacement. If power is lost, DUOPA will not be delivered.
- If the pump is dropped or hit, the battery door or tabs may break. Do not use the pump if the battery door or tabs are damaged because the batteries will not be correctly secured. This may cause loss of power and DUOPA will not be delivered.
- If the pump is dropped or hit, look at the pump for damage. Do not use a pump that is damaged or is not functioning correctly.
- If a gap is present between the battery door and the pump housing, this means the door is not correctly latched. If the battery door becomes detached or loose, the batteries will not be correctly secured. This could cause loss of power and DUOPA will not be delivered.
- Use only DUOPA cassettes for pump accuracy and to make sure the pump works correctly. Attach the DUOPA cassette correctly. A detached or incorrectly attached DUOPA cassette could cause a problem with getting your DUOPA.
CAUTIONS
- Use only Smiths Medical accessories and replacement parts for the pump as using other brands may adversely affect the operation of the pump.
-
Do not operate the pump at temperatures below 36°F (2°C) or above 104°F (40°C).
-
Do not store the pump at temperatures below -4°F (-20°C) or above 140°F (60°C). Do not store the pump with a DUOPA cassette attached. Use the protective cassette provided when storing the pump.
-
Do not keep the pump in humidity levels below 20% or above 90% relative humidity.
-
Do not place the pump in cleaning fluid or water, or allow solution to soak into the pump, keypad, or battery compartment.
-
Do not clean the pump with acetone, other plastic solvents, or abrasive cleaners.
-
Do not use rechargeable NiCd or nickel metal hydride (NiMH) batteries. Do not use carbon zinc (heavy duty) batteries. They do not provide enough power for the pump to operate correctly.
- Do not store the pump for long periods of time with the batteries installed. Battery leakage could damage the pump.
Morning Procedure -
Take the DUOPA carton containing the DUOPA cassettes out of the refrigerator. Check the expiration date on the carton. Do not use any of the cassettes if the expiration date has passed.
-
Take a DUOPA cassette out of the carton. Return the carton with the remaining cassettes to the refrigerator. Do not use the cassette if the expiration date has passed or the cassette is damaged or empty. Leave the DUOPA cassette at room temperature for 20 minutes before using.
- Each DUOPA cassette may be used for up to 16 hours after removal from the refrigerator.
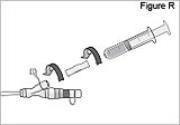
1) Remove the cassette clip (see Figure B): - Remove the cassette tube from its slot in the clip.
- Pull the clip from cassette to slide it off of the cassette top.
2) Attach the DUOPA cassette to the pump (see Figure C): - Hold the pump so the latch faces up.
- Hold the DUOPA cassette so the tube points down.
- Insert the DUOPA cassette hooks into the hinge pins at the base of the pump.
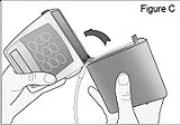
3) Latch the DUOPA cassette into the pump: - Hold the pump and DUOPA cassette upright against a flat surface.
- Press down on the pump, until the DUOPA cassette fits tightly against the pump (see Figure D).
- Use a coin to twist the latch counterclockwise until the latch lines up straight with the arrow (see Figure E).
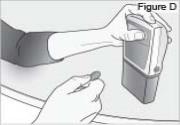

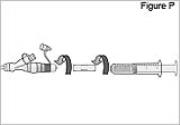
WARNING: Attach the DUOPA cassette correctly. A detached or incorrectly attached cassette could cause a problem with getting your DUOPA. 4) Remove the red cap on the end of the cassette tube (see Figure F). Save the red cap for use when you throw away the cassette.
WARNING: Do not connect the red cap to the stomach tube. It will block DUOPA flow.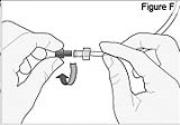
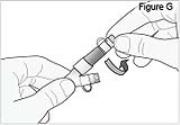
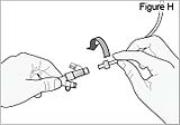
5) Connect the stomach tube to the cassette tube: - While holding the stomach tube steady, twist off the white cap on the end of the longer straight (green) connector (see Figure G). WARNING: Do not twist the stomach tube.
- Connect the cassette tube to the end of the longer straight (green) connector (see Figure H). Do not connect to the shorter angled connector (white, blue, or violet).
6) Turn the pump on:
- Press and hold

until the display turns on.
- Wait approximately 30 seconds for the pump to review settings.
- Check for

on the screen.
PUMP STATUS: The pump is now on but not yet delivering DUOPA.7) Inspect the tubing for kinks or closed clamps. If needed straighten kinks or open clamps (see Figure I). 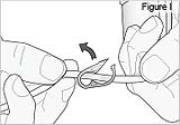
8) Start the pump:
- Press and hold

until 3 dashes appear and then disappear from the screen.
- Wait approximately 15 seconds for the pump to start running.
- Check for

on the display.PUMP STATUS: The pump is now running. DUOPA delivery will begin as programmed by your healthcare provider. If the pump will not start, a message should appear on the display. Refer to the Alarms and Messages section. It will take between 10 minutes and 30 minutes to deliver your morning dose. To start delivery of your Morning Dose you will need to press the Morning Dose key 2 times.
NOTE: If you are unable to deliver your Morning Dose, it may be too soon since the last Morning Dose to deliver another dose. You may need to wait longer. The time between Morning Doses is decided by your healthcare provider.9) The first key press shows the Morning Dose on the display.
- Press

- Check for

on the display. The number on your display is the Morning Dose of DUOPA your healthcare provider prescribed for you.10) The second key press starts Morning Dose delivery.
- Press

a second time to deliver the Morning Dose.
- The display

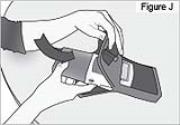
shows a countdown of your Morning Dose.PUMP STATUS: After the Morning Dose finishes, the pump will automatically begin delivering the Continuous Rate. RUN will appear on the display. This completes DUOPA delivery for your Morning Procedure. 11) Insert pump into the carrying bag (see Figure J). - Other carrying cases are also available. Refer to the specific Instructions for Use, which accompanies your carrying case.
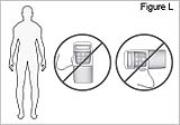
12) Wear the bag over your shoulder or neck: - Place the bag strap over your shoulder or neck (see Figure K).
- Make sure the pump is in correct position (see Figure L).

Extra Dose 1) Give an Extra Dose of DUOPA:
NOTE: If you are unable to deliver the Extra Dose, it may be too soon since the last Extra Dose to deliver another and you may need to wait longer. The time between Extra Doses and the amount of DUOPA in the Extra Dose is decided by your healthcare provider.
- Check for

on the display.
- Press

- Listen for 2 beeps.
- The display will show
PUMP STATUS: The pump is now delivering the Extra Dose. When it finishes, RUN will appear on the display and the Continuous Rate will continue to run. For instructions on changing a DUOPA cassette, see Changing the Cassette.
Evening Procedure You will need: - 1 Syringe
- 1 Syringe connector
- Room temperature water
- 1 Coin, like a quarter
1) Remove the pump from the carrying bag (see Figure M). 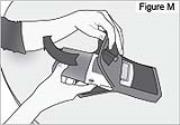
2) Stop the Continuous Rate:
- Press and hold

until 3 dashes appear and then disappear from the display.
- Check for

on the display.3) Turn the pump off:
- Press and hold

until 3 sets of dots appear and then disappear from the display and the display turns off.- Check that the display is off.
4) Clamp the cassette tube (see Figure N). 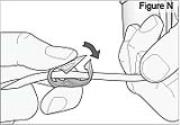
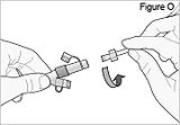
5) Disconnect the tubing: - Twist the cassette tube to disconnect it from the longer straight (green) connector (see Figure O). WARNING: Do not twist the stomach tube.
- Replace the red cap on the cassette tube.
6) Flush the longer straight (green) connector: - Connect the syringe connector to the longer straight (green) connector.
- Fill a syringe with room temperature tap or drinking water. Do not use hot water as it could burn the wall of your stomach or intestine.
- Connect the syringe to the syringe connector (see Figure P). Do not over-tighten the syringe connector or it could break. Do not use the syringe connector if it is cracked or broken.
- Push the syringe plunger to flush the tube. Do not force the syringe if flushing the tube is difficult. Call your healthcare provider if you are unable or have difficulty flushing your tube.
- Remove the syringe and the syringe connector.
- Replace the white cap on the longer straight (green) connector (see Figure Q).
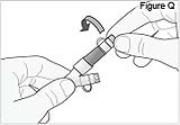
7) Flush the shorter angled connector (white, blue, or violet): - Twist the white cap off the shorter angled connector (white, blue, or violet).
- Connect the syringe connector to the shorter angled connector (white, blue, or violet).
- Fill a syringe with room temperature tap or drinking water. Do not use hot water as it could burn the wall of your stomach or intestine.
- Connect the syringe to the syringe connector (see Figure R). Do not over-tighten the syringe connector or it could break. Do not use the syringe connector if cracked or broken.
- Push the syringe plunger to flush the tube.
- Remove the syringe and the syringe connector.
Replace the white cap on the shorter angled connector (white, blue, or violet) (see Figure S).
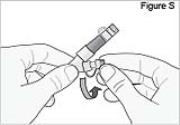
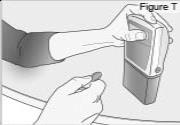
8) Remove the DUOPA cassette from the pump: - Hold the pump and DUOPA cassette upright against a flat surface (see Figure T).
- Use a coin to twist the latch clockwise until the latch pops out (see Figure U).
- Remove the DUOPA cassette from the pump.

Changing the DUOPA Cassette -
Take the DUOPA carton containing the DUOPA cassette out of the refrigerator. Check the expiration date on the carton. Do not use any of the cassettes if the expiration date has passed.
-
Take a DUOPA cassette out of the carton. Return the carton with the remaining cassettes to the refrigerator. Do not use the cassette if the expiration date has passed or the cassette is damaged or empty. Leave the DUOPA cassette at room temperature for 20 minutes before using.
- Each DUOPA cassette may be used for up to 16 hours after removal from the refrigerator.
1) Remove the pump from the carrying bag (see Figure V). 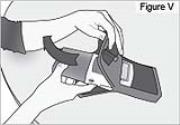
2) Stop the Continuous Rate:
- Press and hold

until 3 dashes appear and then disappear from the display.
- Check for

on the display.3) Turn the pump off:
- Press and hold

until 3 sets of dots appear and then disappear from the display and the display turns off.- Check that the display is off.
4) Clamp the cassette tube (see Figure W). 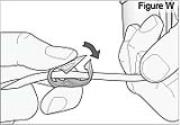
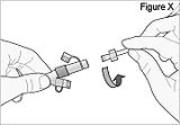
5) Disconnect the tubing: - Twist the cassette tube to disconnect it from the longer straight (green) connector (see Figure X). WARNING: Do not twist the stomach tube.
- Replace the red cap on the cassette tube.
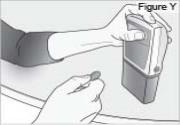
6) Remove the DUOPA cassette from the pump: - Hold the pump and DUOPA cassette upright against a flat surface (see Figure Y).
- Use a coin to twist the latch clockwise until the latch pops out (see Figure Z).
- Remove the DUOPA cassette from the pump.

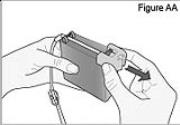
7) Remove the cassette clip on the new DUOPA cassette (see Figure AA): - Remove the cassette tube from its secured slot in the clip.
- Pull the clip from the cassette to slide it off of the cassette top.
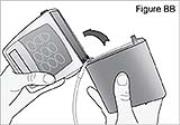
8) Attach the new DUOPA cassette to the pump (see Figure BB): - Hold the pump so that the latch faces up.
- Hold the DUOPA cassette so that the tube points down.
- Insert the DUOPA cassette hooks into the hinge pins at the base of the pump.
9) Latch the new DUOPA cassette into the pump:
- Hold the pump and DUOPA cassette upright against a flat surface.
- Press down on the pump until the DUOPA cassette fits tightly against the pump (see Figure CC).
- Use a coin to twist the latch counterclockwise until the latch lines up straight with the arrow (see Figure DD).
WARNING: Attach the DUOPA cassette correctly. A detached or incorrectly attached cassette could cause a problem with getting your DUOPA.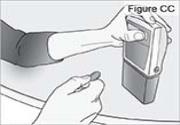
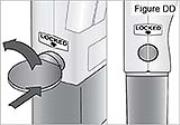
10) Remove the red cap on the end of the cassette tube (see Figure EE).
Save the red cap to use when discarding the cassette.
WARNING: Do not connect the red cap to the stomach tube as it will block DUOPA flow.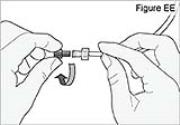
11) Connect the stomach tube to the cassette tube: - While holding the stomach tube steady, twist off the white cap on the end of the longer straight (green) connector (see Figure FF). WARNING: Do not twist the stomach tube.
- Connect the cassette tube to the end of the longer straight (green) connector (see Figure GG). Do not connect to the shorter angled connector (white, blue, or violet).
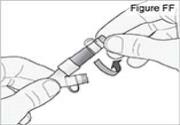
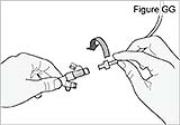
12) Turn the pump on:
- Press and hold

until the display turns on.
- Wait approximately 30 seconds for the pump to review settings.
- Check for

on the display.
PUMP STATUS: The pump is now on but not delivering DUOPA.13) Inspect the tubing for kinks or closed clamps. If needed straighten kinks or open clamps (see Figure HH). 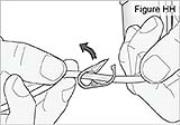
14) Start the pump:
- Press and hold

until 3 dashes appear and then disappear from the display.
- Wait approximately 15 seconds for pump to start running.
- Check for

on the display.
PUMP STATUS: The pump is now running.15) Insert the pump into the carrying bag (See Figure II). 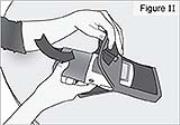
16) Wear the bag on your shoulder or neck: - Place the bag strap over your shoulder or neck (see Figure JJ).
- Make sure the pump is in correct position (see Figure KK).
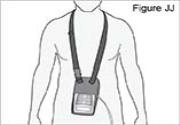
Changing the Batteries:
If you see LowBat or Battery Depleted on the display, change the batteries. Use 2 new AA alkaline batteries such as DURACELL® or EVEREADY® ENERGIZER®. The pump keeps all the important information when the batteries are removed.
WARNING:
-
Always have new batteries available for replacement. If power is lost, DUOPA will not be delivered.
-
If the pump is dropped or hit, the battery door or tabs may break. Do not use the pump if the battery door or tabs are damaged because the batteries will not be correctly secured. This may lead to loss of power and DUOPA will not be delivered.
- If a gap is present anywhere between the battery door and the pump housing, the door is not correctly latched. If the battery door becomes detached or loose, the batteries will not be correctly secured. This could cause loss of power and DUOPA will not be delivered.
CAUTION:-
Do not use rechargeable NiCd or nickel metal hydride (NiMH) batteries. Do not use carbon zinc (“heavy duty”) batteries. They do not provide enough power for the pump to operate correctly.
- Do not store the pump for prolonged periods of time with the batteries installed. Battery leakage could damage the pump.
1) Ensure the pump is stopped. 2) Push and hold the arrow button while sliding the battery door until it comes completely off the pump (see Figure LL). 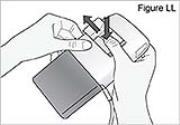
3) Remove the used batteries (see Figure MM). 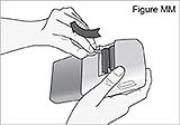
4) Install new batteries into the battery compartment.
NOTE: Insert the batteries correctly based on the picture in the battery compartment. If you insert the batteries backwards, the display will remain blank. Reinsert the batteries, making sure to match the + and – markings with the battery compartment picture.5) Listen for a beep.
PUMP STATUS: The pump is now powered. The power-up sequence will start, the pump will go through an electronic self-test, and then the pump will beep 6 times at the end of the power-up sequence. All of the display indicators, the software revision, and each setting will appear briefly.
If you do not hear a beep, and the display is off, the pump is not powered. Check that the batteries are correctly inserted.6) Slide the battery door back onto the pump into its original closed position (see Figure NN). 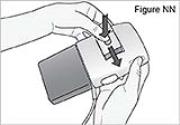
Change the Morning Dose Your healthcare provider may have set your pump to allow for dose changes to your Morning Dose and Continuous Rate (Lock Level 1). Do not change your medicine dose without approval and training from your healthcare provider.
Talk with your healthcare provider to decide when to change your Morning Dose and Continuous Rate. Do not change your Extra Dose unless your healthcare provider tells you to. If your Extra Dose requires changes, your healthcare provider will provide instructions.Change the Morning Dose WARNING: Do not use the Prime button. Priming is for use by your healthcare provider only. 1) Turn the pump on:
- Press and hold

until the display turns on.
- Wait approximately 30 seconds for pump to review settings.
- Check for

on the display.
PUMP STATUS: The pump is now on but not yet delivering DUOPA.2) Inspect the tubing for kinks or closed clamps. If needed, straighten kinks or open clamps (see Figure OO). 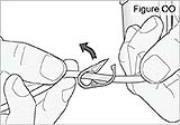
3) Start the pump:
- Press and hold

until 3 dashes appear and then disappear from the display.
- Wait approximately 15 seconds for pump to start running.
- Check for

on the display.
PUMP STATUS: The pump is now running.4) Change the Morning Dose:
a. Press

- time.

on the display.
c. Press

or

to select the desired Morning Dose.
d. Press

to store the Morning Dose.
e. Make sure you see the correct Morning Dose on the display. If not, repeat Steps 4c to 4e.5) Deliver the Morning Dose:
- Press

1 time.
NOTE: If you see “Value not saved” on the display, press NEXT and then repeat Steps 4c to 4e.
- The display

shows a countdown of your Morning Dose.
PUMP STATUS: After the Morning Dose finishes, the pump will begin delivering the Continuous Rate. RUN will appear on the display.
NOTE: If you are unable to deliver a Morning Dose, it may be too soon since the last Morning Dose to deliver another and you may need to wait longer. The time between Morning Doses is decided by your healthcare provider.Change the Continuous Rate 1) Stop the Continuous Rate:
- Press and hold

until 3 dashes appear and then disappear from the display.
- Check for

on the display.2) Change the Continuous Rate:
a. Press

2 times.
b. Check for

on the display.
c. Press

or

to select the desired Continuous Rate.
d. Press

to store the Continuous Rate.
e. Make sure you see the desired Continuous Rate on the display. If not, repeat Steps 2c to 2e.3) Start the pump:
- Press and hold

until 3 dashes appear and then disappear from the display.
NOTE: If you see “Value not saved” on the display, press NEXT and then repeat Steps 2c to 2e.
- Wait approximately 15 seconds for the pump to start running.
- The display will show

PUMP STATUS: The pump is now running.Alarms and Messages
The table below shows some of the common alarms that you may hear from the pump. With all alarms, read the display before pressing

to silence the alarm.What you see: What you hear: Meaning Response Error Two-Tone Alarm An error with the pump has occurred. Contact your healthcare provider. High Pressure Two-Tone Alarm There is pressure backed up in the tubing. Check tubing for clamps, kinks, or blockages. Make sure the red cap has been removed from the DUOPA cassette tube. Flush connectors if necessary. If it is not possible to flush the tubes, contact your healthcare provider as your tube may be blocked. LowBat 3 Two-Tone Beeps Every 5 minutes The pump batteries are low. Change the batteries right away. Upstream Occlusion Two-Tone Alarm If your healthcare provider has the Upstream Occlusion Sensor set to ON and a blockage in the DUOPA cassette is detected, this alarm will sound. Detach the DUOPA cassette. Check if the DUOPA cassette is empty. If not empty, reattach the DUOPA cassette. Restart the pump to continue delivery. Contact your healthcare provider if the alarm continues. No message on display Two-Tone Alarm Batteries were removed within approximately 15 seconds after stopping the pump. Install new batteries to silence the alarm. Otherwise, the alarm will stop within a short period of time. Display shows current pump status 2 Beeps (Long-Short) The DUOPA cassette is not lined up with the pump or DUOPA is not flowing from the DUOPA cassette to the pumping mechanism.
Very cold or extremely thick DUOPA may cause this alarm as well.Press NEXT to silence the alarm. The pump continues to run. Make sure the DUOPA cassette is correctly lined up with the pump and DUOPA is flowing.
Take the DUOPA cassette out of the refrigerator for 20 minutes before attaching to the pump.Battery Depleted Two-Tone Alarm Batteries are dead. Install new batteries. To continue delivery, restart the pump when completed. Key pressed, Please release Two-Tone Alarm Key is being held down. Stop pressing key. If the alarm persists, close the cassette tube clamp and remove the pump from use. Contact your healthcare provider. No Disposable, Clamp Tubing Two-Tone Alarm Disposable refers to the DUOPA Cassette. No Disposable means the DUOPA cassette was removed. The pump is not sensing proper cassette attachment. Clamp the cassette tube and disconnect it from your stomach tube. A DUOPA cassette must be correctly attached in order for the pump to run. Press NEXT to silence the alarm. No Disposable, Pump won't run Two-Tone Alarm Disposable refers to the DUOPA Cassette. You have tried to start the pump without a disposable DUOPA cassette attached. Press NEXT to silence the alarm. A DUOPA cassette must be correctly attached for the pump to run. Service Due See manual Two-Tone Alarm The pump is scheduled for service. Press NEXT to silence the alarm. The pump is still working, but contact your healthcare provider for further instructions. Frequently Asked Questions What if I drop the pump or hit it against a hard surface?
Do the following right away:
- Check the DUOPA cassette latch on the side of the pump and make sure the line on the latch lines up with the arrow on the side of the pump.
- Gently twist, push, and pull on the DUOPA cassette to make sure it is still firmly attached.
- Check the battery door to make sure it is still firmly attached.
Stop the pump right away, close the tubing clamp, and contact your healthcare provider.What should I do if I drop the pump in water?
If you accidentally drop the pump in water, pick it up quickly, dry it off with a towel, and call your healthcare provider.WARNING: If the pump is dropped or hit, look at the pump for damage. Do not use a pump that is damaged or is not working correctly. What should I do if I need to bathe while wearing the pump?
You’ll need to detach the pump before you shower, bathe, or swim. Reattach the pump to the stomach tubing afterwards and restart it.What should I do if I need to have a medical test while wearing the pump?
The pump may need to be removed prior to certain medical tests. Be sure to talk to your doctor about your DUOPA pump before you take these tests.STORAGE and DISPOSAL Storage - Store DUOPA in the refrigerator with the temperature between 36°F to 46°F (2°C to 8°C).
- When the DUOPA cassette has been removed from the refrigerator, DUOPA should be used within 16 hours.
- The DUOPA cassettes are for single use only and should not be used for longer than 16 hours, even if some of the medicine remains. An opened cassette should not be re-used.
- Protect the cassette from light and keep it in the carton before using.
Throwing away your DUOPA cassette or batteries - Throw away the DUOPA cassette as your healthcare provider tells you to.
- Throw away used batteries in a manner safe for the environment, and according to any regulations that apply.
This Instructions for Use has been approved by the U.S. Food and Drug Administration.

AbbVie Inc.
North Chicago, IL 60064, U.S.A.
For DUOPA Support: 1-844-386-4968
Pump manufactured by:
Smiths Medical ASD, Inc.
1265 Grey Fox Road
St. Paul, MN 55112 USA
Tel: 1-800-258-5361
www.smiths-medical.com03-B884 Revised: March 2019
Legacy 1400 Operator's Manual
CADD-Legacy® 1400 Pump
Model 1400This online version differs from the printed version. Certain information that is not intended for patients has been removed.
This operator’s manual is for clinician use only. Read the entire operator’s manual before operating the pump.
This manual pertains only to the CADD-Legacy® 1400 pump. There are other CADD-Legacy® pump models available; review the rear label of the pump to ensure it is a CADD-Legacy® 1400 pump before programming. This pump is designed for enteral delivery of medication and can be programmed to deliver a continuous rate, a morning dose, and extra doses.
This manual is intended for clinician use only. Do not permit patients to have access to this manual. The pump has 3 security levels designed to limit patient access. Do not disclose the pump’s security codes or any other information that would allow inappropriate access to programming and operating functions.
The issue date of this operator’s manual is included on the back cover for the clinician’s information. In the event one year has elapsed between the issue date and product use, the clinician should contact Smiths Medical to see if a later revision of this manual is available.
Technical Assistance
If you have comments or questions concerning the operation of the CADD-Legacy® 1400 pump, please call the number given below. When calling, please specify the pump’s software revision. This information is located on the pump’s display during power up.
Our staff at Smiths Medical is available to help clinicians 24 hours a day with the programming and operation of the CADD-Legacy® 1400 pump.
Smiths Medical ASD, Inc.
1265 Grey Fox Road
St. Paul, MN 55112 USA
Tel: 1 800 258 5361 (USA)
Tel: +1 614 210 7300
www.smiths-medical.comRead this entire operator’s manual before operating the CADD-Legacy® 1400 pump.
Failure to follow the warnings and cautions below could result in return of symptoms, damage to the pump, serious injury, or death in extreme cases.
Please refer to the full prescribing information for DUOPA (carbidopa and levodopa) enteral suspension for indications and usage, contraindications, warnings, precautions, and adverse reactions.
Warnings
- This operator’s manual should be used by clinicians only. Do not permit patients to have access to this manual, as the information contained would allow the patient complete access to all programming and operating functions. Improper programming could compromise patient treatment.
- To avoid explosion hazard, do not use the pump in the presence of flammable anesthetics or explosive gases.
- The CADD-Legacy® 1400 pump and medication cassette reservoir are designed for enteral delivery of medication only. They are NOT intended for IV or other parenteral routes of infusion.
- Do not disclose to the patient the pump’s security codes or any other information that would allow the patient complete access to all programming and operating functions. Improper programming could compromise patient treatment.
- Always have new batteries available for replacement. If power is lost, non-delivery of medication will occur.
- If the pump is dropped or hit, the battery door or tabs may break. Do not use the pump if the battery door or tabs are damaged because the batteries will not be properly secured; this may result in loss of power and non-delivery of medication.
- If a gap is present anywhere between the battery door and the pump housing, the door is not properly latched. If the battery door becomes detached or loose, the batteries will not be properly secured; this could result in loss of power and non-delivery of medication.
- System delivery inaccuracies beyond the stated accuracy may occur as a result of back pressure or fluid resistance, which depends upon temperature, medication viscosity, catheter size, extension set tubing, flow rate, and orientation of the pump system.
- Programming the pump at a delivery rate other than what is prescribed will cause over- or under-delivery of medication.
- Clamp the fluid path tubing and/or disconnect the tubing from the enteral access device before removing the medication cassette reservoir from the pump to prevent unintended delivery of medication.
- Use only approved DUOPA medication cassette reservoirs to maintain pump accuracy and assure proper pump operations.
- Use only extension sets approved for use with DUOPA, paying particular attention to all warnings and cautions associated with their use.
- Attach the cassette properly. The cassette is the part of the medication cassette reservoir that attaches to the pump. A detached or improperly attached cassette could result in unintended delivery of medication.
- Do not prime the fluid path with the tubing connected to a patient as this could result in over-delivery of medication.
- If the pump is dropped or hit, inspect the pump for damage. Do not use a pump that is damaged or is not functioning properly, as this could compromise patient treatment.
- The use of power supplies and a remote dose cord other than those listed in the electromagnetic emissions declaration may result in increased emissions or decreased immunity of the pump.
- The pump should not be used adjacent to or stacked with other equipment. If adjacent or stacked use is necessary, the user should verify normal operation of the pump in the configuration in which it is to be used.
- There are potential health hazards associated with improper disposal of batteries, electronics, and contaminated (used) reservoirs and extension sets. Dispose of used batteries, reservoirs, extension sets and other used accessories, or a pump that has reached the end of its useful life, in an environmentally safe manner, and according to any regulations that may apply.
Cautions
- Use only Smiths Medical accessories and replacement parts, as using other brands may adversely affect the operation of the pump.
- Do not operate the pump at temperatures below 2°C (36°F) or above 40°C (104°F).
- Do not store the pump at temperatures below -20°C (-4°F) or above 60°C (140°F). Do not store the pump with the medication cassette reservoir attached. Use the protective cassette provided.
- Do not expose the pump to humidity levels below 20% or above 90% relative humidity.
- Do not sterilize the pump or medication cassette reservoir as this could cause damage.
- When the upstream occlusion sensor is turned off, the pump will not detect occlusions in the medication cassette reservoir. Periodically inspect the medication cassette reservoir for decreasing volume. Undetected occlusions could result in under- or non-delivery of medication.
- Do not use rechargeable NiCd or nickel metal hydride (NiMH) batteries. Do not use carbon zinc (“heavy duty”) batteries. They do not provide sufficient power for the pump to operate properly.
- Do not store the pump for prolonged periods of time with the batteries installed. Battery leakage could damage the pump.
- Prior to starting medication delivery, inspect the fluid path for kinks, a closed clamp, or other obstruction. An undetected occlusion may result in under- or non-delivery of medication and/or nuisance alarms.
- When you enter a new value, any lockout time already in effect will be cleared. An extra dose or morning dose could be requested and delivered immediately upon starting the pump, which may result in over-delivery of medication.
- Do not immerse the pump in cleaning fluid or water. Do not allow solution to soak into the pump, accumulate on the keypad, or enter the battery compartment. Moisture buildup inside the pump may damage the pump.
- Do not clean the pump with acetone, other plastic solvents, or abrasive cleaners, as damage to the pump may occur.
- Do not expose the pump to therapeutic levels of ionizing radiation as permanent damage to the pump’s electronic circuitry may occur. The best procedure to follow is to remove the pump from the patient during therapeutic radiation sessions. If the pump must remain in the vicinity during a therapy session, it should be shielded, and its ability to function properly should be confirmed following treatment.
- Do not expose the pump directly to ultrasound, as permanent damage to the electronic circuitry may occur.
- Do not use the pump in the vicinity of magnetic resonance imaging (MRI) equipment as magnetic fields may adversely affect the operation of the pump. Remove the pump from the patient during MRI procedures and keep it at a safe distance from magnetic energy.
- This pump may interfere with ECG equipment. Monitor ECG equipment carefully when using this pump.
- CADD-Legacy® 1400 pumps are sealed units. A broken or damaged seal will, therefore, be considered conclusive evidence that the pump has been misused and/or altered, which voids any and all warranties. All service and repair of CADD-Legacy® 1400 pumps must be performed by Smiths Medical or its authorized agents.
- Review programming screens when complete to make sure desired programming has been entered. Check to make sure unintended changes were not made to the morning dose, continuous rate, or extra dose volume. If unintended changes were made, go to the appropriate screen and program the desired value.
Table of Contents Warnings Cautions 1.0 General Description Introduction Indications Symbols Pump Diagram Description of the Keys, Display, and Features The Main Screen Lock Levels Security Codes Lock Level 0 (LL0) Table Lock Levels 1 and 2 (LL1, LL2) Table 2.0 Pump Setup and Programming Installing or Replacing the Batteries Watching Power Up Changing to Lock Level 0 (LL0) Programming the Pump: General Instructions Delivery Methods Programming Screens Programming Delivery Programming a Morning Dose Removing a Medication Cassette Reservoir Attaching a Medication Cassette Reservoir Priming the Tubing and Connecting to the Patient Setting the Lock Level for the Patient Programming with Upper Limits, Adjusting Doses in Lock Level 1 3.0 Operating the Pump Starting the Pump Stopping the Pump Turning the Pump On/Off Starting a Morning Dose Starting an Extra Dose Stopping an Extra Dose or Morning Dose Resetting the Reservoir Volume 4.0 Biomed Functions Overview: Accessing the Biomed Functions and Programming the Lockouts Extra Dose Lockout Morning Dose Lockout Upstream Occlusion Sensor On/Off 5.0 Reference Messages and Alarms, Alphabetical List What if the pump is dropped or hit? Cleaning the Pump and Accessories Exposure to Radiation, Ultrasound, Magnetic Resonance Imaging (MRI), or Use near ECG Equipment Continuous Rate Scroll Ranges Extra Dose, Morning Dose Scroll Ranges Technical Description Specifications (Nominal) Accuracy Test Results Electromagnetic Emissions and Immunity Declarations Safety Features and Fault Detection Software Safety Features Data Handling Software Safety Features Annual Functional Inspection Collect Separately Limited Warranty Index Appendix A – Pump Programming Quick Reference for Healthcare Providers 1.0 General Description
Introduction
The CADD-Legacy® 1400 pump provides enteral delivery of medication to patients in hospital or outpatient settings. Therapy should always be overseen by a physician or a certified, licensed healthcare professional. As appropriate to the situation, the patient should be instructed in using and troubleshooting the pump.
Indications
The CADD-Legacy® 1400 pump is indicated solely for the enteral delivery of medication contained in a medication cassette reservoir supplied by AbbVie. The medication cassette reservoir attaches to the bottom of the pump.
WARNING: The CADD-Legacy® 1400 pump and medication cassette reservoir are designed for enteral delivery of medication only. They are NOT intended for IV or other parenteral routes of infusion. Use of this product for medications or therapies outside the intended use can result in death or serious patient injury. Refer to AbbVie’s full prescribing information for DUOPA (carbidopa and levodopa) enteral suspension for indications and usage, contraindications, warnings, precautions, and adverse reactions.
Symbols

Direct current (power jack) 
Accessory jack 
Caution 
Class II equipment 
Type CF equipment 
Splashproof – water splashed against the pump housing will have no harmful effects (see Cleaning the Pump and Accessories, Section 5, for additional important information). 
Catalog number 
Serial number 
Date of manufacture 
Manufacturer 
CAUTION: Federal (USA) law restricts this device to sale by or on the order of a physician. 
Collect separately 
Temperature limitation 
Humidity limitation 
Atmospheric pressure limitation 
MR Unsafe Pump Diagram
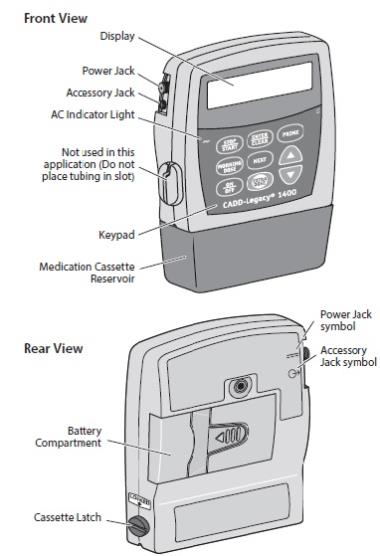
Description of the Keys, Display, and Features
AC Indicator Light
The green indicator light is on when you are using the AC adapter to power the pump.
Display
The liquid crystal display (LCD) shows programming information and messages. In this manual, the term “display” is synonymous with display panel or LCD.
Keypad
The keys on the keypad are described below. A key beeps when pressed if it is operable in the current lock level.

used to start and stop pump delivery; silences alarms. 
used to enter (save) a new value in the pump’s memory when programming doses or pump settings or to clear values from record-keeping screens. It is also used to return from the biomed functions to the main screen (see Section 4). 
used to fill the tubing with medication. 
used to confirm and deliver the morning dose (typically used as a daily loading dose) when the pump is running. When the pump is stopped, it is used to view or change the pump’s current lock level. Lock levels are used to limit patient access to certain programming and operating functions. (See Lock Levels, this section.) 
used to move from one programming screen to the next without changing the setting or value displayed; silences alarms. 
used to “scroll up” or increase a value, or scroll through biomed function settings. 
used to “scroll down” or decrease a value, or scroll through biomed function settings. 
used to put the pump into a low power state when not in use or back into full power. 
used by the patient to deliver a programmed amount of medication upon request (extra dose). Power Jack
You may plug an AC adapter into the power jack as an alternate source of power. The indicator light on the front of the pump will illuminate when the AC adapter is in use.
Accessory Jack
The accessory jack is used for attaching accessory cables. See the instructions for use supplied with those accessories.
Medication Cassette Reservoir
The medication cassette reservoir is the single-use reservoir designed for use with the CADD-Legacy® 1400 pump. In this manual and on the pump’s display, the word “disposable” refers to the medication cassette reservoir. In AbbVie’s patient instructions for use, medication cassette reservoir is referred to as DUOPA cassette.
Battery Compartment
Two AA batteries fit into the battery compartment. The AA batteries serve as the primary source of power, or as backup power when an AC adapter is in use.
Cassette Latch
The cassette latch attaches the cassette to the pump. The term “cassette” refers to the part of the medication cassette reservoir that attaches to the bottom of the pump. If the cassette becomes unlatched while the pump is running, delivery will stop and an alarm will occur. If the cassette becomes unlatched while the pump is stopped, an alarm will occur.
Other Features Not Shown
Upstream Occlusion Sensor: The pump contains an upstream occlusion sensor. This feature may be turned on or off (see Section 4, Biomed Functions). When the sensor is turned on, and an occlusion in the reservoir is detected, an alarm will sound, delivery will stop, and the display will show Upstream Occlusion.
CAUTION: When the upstream occlusion sensor is turned off, the pump will not detect occlusions in the medication cassette reservoir. Periodically inspect the medication cassette reservoir for decreasing volume. Undetected occlusions could result in under- or non-delivery of medication. Downstream Occlusion Sensor: The pump contains a downstream occlusion sensor. When a downstream occlusion (between the pump and the patient) is detected, an alarm will sound, delivery will stop, and the display will show High Pressure.
Reservoir Volume Alarm: The reservoir volume alarm indicates when the volume of medication in the medication cassette reservoir is low or depleted. Each time you change the medication cassette reservoir, you may reset the reservoir volume to the originally programmed value. Then, as medication is delivered, the reservoir volume automatically decreases. When the pump calculates that 5 ml remain in the medication cassette reservoir, beeps sound and ResVol Low appears on the main screen. This alarm recurs at every subsequent decrease of 1 ml until the reservoir volume reaches 0 ml, at which point the pump stops and the reservoir volume empty alarm sounds.
NOTE: The default setting for Reservoir Volume is Not in Use. The reservoir volume alarm is activated only when a value is programmed into the Reservoir Volume screen. Programming a reservoir volume value is not required for general use, but is available at provider discretion.
The Main Screen
The main screen is the starting point for programming or viewing the pump’s settings.
If no keys are pressed for 2 minutes, the display reverts to the main screen. When the two AA batteries are low, LowBat appears on the main screen.
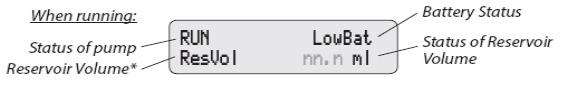
*Does not appear on the main screen if reservoir volume is programmed to Not In Use.
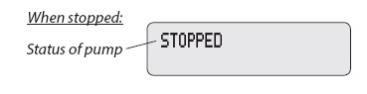
Lock Levels
Lock levels are used to limit patient access to certain programming and operating functions. The table on the next page lists the functions that are accessible in lock level 0 (LL0), lock level 1 (LL1), and lock level 2 (LL2). When a function is accessible, the key associated with the function beeps when pressed. If a function is not accessible, the pump ignores the key press and a beep does not sound. Section 2, Pump Setup and Programming, describes how to change the lock level.
Security Codes
The following security codes are preset by the manufacturer for the clinician’s use:
**Text Omitted**
WARNING: Do not disclose to the patient the pump’s security codes or any other information that would allow the patient complete access to all programming and operating functions. Improper programming could compromise patient treatment. Lock Level 0 (LL0) Table
This table lists the operations that are accessible in lock level 0 (LL0) while the pump is stopped and running. LL0 permits complete access to all programming and operating functions.
Stopped Running Pump Operations and Programming LL0 Any Lock Level Stop/Start the pump Yes Yes Reset reservoir volume Yes No Prime Yes No Change the lock level Yes, w/code No Change morning dose No Yes Start an extra dose No Yes Start a morning dose No Yes Change continuous rate Yes No Change extra dose Yes No Clear given amount Yes No Biomed Functions Access to functions Yes, w/code No Extra dose lockout Yes, w/code No Morning dose lockout Yes, w/code No Upstream occlusion Sensor On/Off Yes, w/code No Lock Levels 1 and 2 (LL1, LL2) Table
This table lists the operations that are accessible in lock level 1 (LL1) and lock level 2 (LL2) while the pump is stopped and running. LL1 permits limited control of pump programming and operations. LL2 permits only minimal control of pump operations.
Pump Operations and Programming Stopped Running LL1 LL2 Any Lock Level Stop/Start the pump Yes Yes Yes Reset reservoir volume Yes Yes No Prime Yes No No Change the lock level Yes, w/code Yes, w/code No Change morning dose No No Yes* Start an extra dose No No Yes Start a morning dose No No Yes Change continuous rate Yes* No No Change extra dose Yes* No No Clear given amount Yes No No *When in LL1, you can program up to the LL0 value. No programming is allowed in LL2.
2.0 Pump Setup and Programming
Installing or Replacing the Batteries
Use new, AA alkaline batteries such as DURACELL® or EVEREADY® ENERGIZER® batteries to power the pump. The pump retains all programmed values while the batteries are removed.
Dispose of used batteries in an environmentally safe manner, and according to any regulations which may apply.
WARNING: - Always have new batteries available for replacement. If power is lost, non-delivery of medication will occur, which could compromise patient treatment.
- If the pump is dropped or hit, the battery door or tabs may break. Do not use the pump if the battery door or tabs are damaged because the batteries will not be properly secured; this may result in loss of power and non-delivery of medication, which could compromise patient treatment.
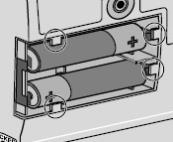
CAUTION: Do not use rechargeable NiCd or nickel metal hydride (NiMH) batteries. Do not use carbon zinc (“heavy duty”) batteries. They do not provide sufficient power for the pump to operate properly. In order to install or replace the batteries, be sure the pump is Stopped. Then, follow these steps:
1. Push down and hold the arrow button while sliding the door off.
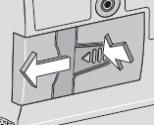
2. Remove the used batteries. Pulling on the end of the battery strap will make battery removal easier.
3. Install the new batteries in the compartment, making sure the battery strap is positioned correctly under the batteries.
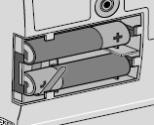
NOTE:
-
Be sure to match the polarity markings of the new batteries (+ and –) with those labeled in the battery compartment. If you put the batteries in backwards, the display will remain blank, and you will not hear a beep.
- Use two new, AA alkaline batteries to power the pump. You may use any alkaline batteries, including DURACELL® Alkaline and EVEREADY® ENERGIZER® Alkaline, for example.
4. Place the battery door over the battery compartment and slide the door closed.
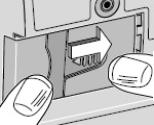
5. Ensure that the door is latched by trying to remove the door without pressing the arrow button.
NOTE: The power-up sequence will start, the pump will go through an electronic self-test, and the pump will beep 6 times at the end of the power-up sequence. All of the display indicators, the software revision, and each parameter will appear briefly.
WARNING: If a gap is present anywhere between the battery door and the pump housing, the door is not properly latched. If the battery door becomes detached or loose, the batteries will not be properly secured; this could result in loss of power and non-delivery of medication, which could compromise patient treatment. 
6. Resume operation of the current program by pressing and holding

to start the pump or proceed to program the pump.
NOTE:
-
The life of the batteries is dependent on the amount of medication delivered, delivery rate, battery age, and the temperature.
-
At the rate of 100 ml per day, alkaline batteries will usually last about 7 days.
- The power of the batteries will be quickly depleted at temperatures below 10°C (50°F).
CAUTION: Do not store the pump for prolonged periods of time with the batteries installed. Battery leakage could damage the pump. Watching Power Up
When you install the batteries, the pump will start its power up sequence during which it performs self-tests and displays programmed values. Watch for the following:
- Pump model number and last error code (“LEC”) if any, will appear. (If an error code appears, the pump should be removed from use and returned for service.)
- The software revision will appear.
- The display will turn on, showing a series of blocks. Look for any blank areas, which would indicate a faulty display.
- The display will turn off briefly.
- The pump’s program screens will appear, followed by the current lock level setting. The pump will beep after each screen. If messages appear, see Messages and Alarms Table, Section 5 for further explanation and instruction.
- When power up is complete, 6 beeps will sound, and the pump will be stopped on the main screen.
NOTE: To move quickly through the power-up screens, press

repeatedly. To skip the automatic review entirely, press

If you attempt to skip screens before the pump is powered up, it will not respond.
Changing to Lock Level 0 (LL0)
Before programming the pump, make sure the pump is set to LL0. LL0 allows the clinician to access all programming and operating functions.
- Make sure the pump is stopped. Press

The current lock level will appear. (If the lock level is already LL0, press

to exit.)
**Text Omitted**
WARNING: Do not disclose to the patient the pump’s security codes or any other information that would allow the patient complete access to all programming and operating functions. Improper programming could compromise patient treatment. Programming the Pump: General Instructions
WARNING: System delivery inaccuracies beyond the stated accuracy may occur as a result of back pressure or fluid resistance, which depends upon temperature, medication viscosity, catheter size, extension set tubing, and orientation of the pump system. The procedure for changing a programmed setting is similar for most programming screens.
- Make sure the pump is stopped and in lock level 0.
- To begin programming, start at the main screen and press

- To change a setting, press

or

until the desired setting appears. (Press and hold these keys to change values with increasing speed.)
- Press

within 25 seconds to confirm a change or the screen will revert to the previous setting.
- If any key other than

is pressed, Value not saved will appear. Press

to return to the screen being programmed, scroll to the desired value, and press

- Press

to advance to the next screen.
- To leave a setting unchanged, press

to go to the next screen.
Delivery Methods
WARNING: Programming the pump at a delivery rate other than what is prescribed will cause over- or under-delivery of medication, which could compromise patient treatment. Please refer to the prescribing information for DUOPA for dosage and administration information. The CADD-Legacy® 1400 pump offers 3 methods of delivery:
- Continuous rate
- Extra dose
- Morning dose
The following graph illustrates the combined delivery methods. The continuous rate, extra dose, and morning dose are programmed as described in this section. Ranges and programming increments are listed in the Specifications in Section 5.
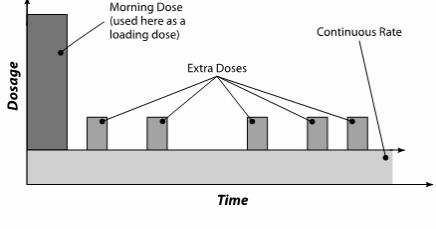
Programming Screens
These are the programming screens for the CADD-Legacy® 1400 pump. Descriptions of the screens follow.
Reservoir Volume (ml) 
Continuous Rate (ml/hr) 
Extra Dose (ml) 
Given (ml) 
Reservoir Volume
NOTE: The default setting for Reservoir Volume is Not in Use. Programming a reservoir volume value is not required for general use, but is available at provider discretion.
If you wish to use the reservoir volume feature, enter the volume of medication contained in the filled medication cassette reservoir. The reservoir volume value decreases as the pump delivers medication or as you prime the tubing. When you change the medication cassette reservoir, reset the reservoir volume value on this screen. If you do not wish to use the reservoir volume feature, scroll down to Not In Use (located before 1 and after 9999 in the range of values).
The reservoir volume value could be set higher than the capacity of the medication cassette reservoir. Be sure to program the reservoir volume to reflect the actual volume in the reservoir.
Continuous Rate
Enter the continuous rate of medication delivery in ml/hr. The maximum rate is 20 ml/hr. If the prescription does not call for a continuous rate, enter zero.
NOTE: If you intend to run the pump in lock level 1 so the continuous rate can be varied, you should enter the maximum allowable rate while programming in lock level 0. After programming, you may then change to lock level 1 and decrease the rate to its starting value. See Programming with Upper Limits, Adjusting Doses in LL1 at the end of Section 2.
Extra Dose
Enter the amount of medication to be delivered when the patient presses

If the prescription does not call for an extra dose, enter zero.
NOTE: If you intend to run the pump in lock level 1 so the extra dose can be varied, you should enter the maximum allowable dose while programming in lock level 0. After programming, you may then change to lock level 1 and decrease the dose to its starting value. See Programming with Upper Limits, Adjusting Doses in LL1 at the end of Section 2.
Given
This screen shows the total amount of medication delivered since the last time this value was cleared. The amount shown is rounded to the nearest 0.05 ml. If this value reaches 99999.95, it automatically returns to 0 and continues counting. When using the pump’s

key, the amount of medication used is not included in the Given amount.
Other Programming Information
The morning dose should be programmed separately following programming of the above. Information on programming the morning dose can be found later in this section.
Programming Delivery
WARNING: Programming the pump at a delivery rate other than what is prescribed will cause over- or under-delivery of medication, which could compromise patient treatment. Please refer to the prescribing information for DUOPA for dosage and administration information. To program the pump, enter the prescribed values.
1. Begin at the main screen.
- Make sure the pump is in LL0.
- Make sure STOPPED appears on the main screen.
- Press

to begin.
2. Enter the reservoir volume (optional – not required for general use).
NOTE: The default setting for Reservoir Volume is Not in Use. Programming a reservoir volume value is not required for general use, but is available at provider discretion.
- Press

or

to select the volume in the filled medication cassette reservoir. (If you do not wish to use the reservoir volume feature, scroll down to Not In Use located before 1 or after 9999.)
- Press

- Press

3. Enter the continuous rate.
- Press

or

to select the desired continuous rate.
- Press

- Press

4. Enter the extra dose amount.
- Press

or

to select the desired extra dose amount.
- Press

- Press

NOTE: If required, program the extra dose lockout time, as instructed in Section 4, Biomed Functions.
5. Clear the amount given.
- Press

if you wish to clear the amount given.
- Press

6. Review the program.
Press

repeatedly to review the programming screens. If you need to reprogram a setting, press

until the appropriate screen appears and change the setting as described in this section.
Programming a Morning Dose
To program a morning dose the pump must be running and a medication cassette reservoir must be attached.
To program a morning dose
1. Make sure the pump is running and in LL0 or LL1. Start the pump, if necessary.
NOTE: In LL0, programming in the full range is possible. In LL1, you can program up to the LL0 value.2. Press

The current morning dose value will appear.
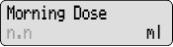
3. Press

or

to select the desired morning dose value.
4. Press

to enter the value into the pump’s memory. (If desired, press

again to begin delivering the morning dose.)
Once entered, the morning dose amount is retained in the pump’s memory. The patient can then press

twice to display and deliver the morning dose.
NOTE: If required, program the morning dose lockout time, as instructed in Section 4, Biomed Functions.
Removing a Medication Cassette Reservoir
WARNING: Clamp the fluid path tubing and/or disconnect the tubing from the enteral access device before removing the medication cassette reservoir from the pump to prevent uncontrolled delivery of medication, which could compromise patient treatment. To remove the medication cassette reservoir from the pump
1. Stop the pump.
2. Close the tubing clamp. If necessary, disconnect the tubing from the enteral access device.
3. Insert a coin into the slot in the cassette latch and turn it clockwise. The latch will pop out when you unlatch the cassette (the part of the medication cassette reservoir that attaches to the bottom of the pump).
4. A continuous alarm will sound and the pump will display No Disposable, Clamp Tubing (the pump is not sensing proper cassette attachment). The alarm may be silenced by pressing

or

5. Remove the cassette hooks from the pump hinge pins.
Attaching a Medication Cassette Reservoir
Obtain a new, filled medication cassette reservoir.
WARNING: -
Use only approved DUOPA medication cassette reservoirs to maintain pump accuracy and assure proper pump operations.
- Use only extension sets approved for use with DUOPA, paying particular attention to all warnings and cautions associated with their use.
After attaching the medication cassette reservoir, proceed to the reservoir volume screen to reset the value for the volume, and then prime the tubing.
To attach the medication cassette reservoir to the pump 1. Clamp the tubing.
2. Insert the cassette hooks into the hinge pins on the pump.
3. Place the pump upright on a firm, flat surface. Press down so the cassette fits tightly against the pump.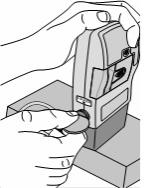
4. Insert a coin into the slot in the cassette latch, push in, and turn counterclockwise until the line on the latch lines up with the arrow on the side of the pump and you feel the latch click into place. 
WARNING: Attach the cassette properly. The cassette is the part of the medication cassette reservoir that attaches to the pump. A detached or improperly attached cassette could result in unintended delivery of medication, which could compromise patient treatment. 5. Gently twist, push, and pull on the medication cassette reservoir to make sure it is firmly attached. If the cassette is not secure, repeat the procedure from step 1. 
Priming the Tubing and Connecting to the Patient
WARNING: Do not prime the fluid path with the tubing connected to a patient as this could result in over-delivery of medication, which could compromise patient treatment. The pump must be stopped and in LL0 or LL1 in order to prime the fluid path. If the pump is in LL2, you cannot prime the fluid path.
NOTE: If you are not changing the medication cassette reservoir but wish to prime the fluid path, you may follow the same procedure.
1. Make sure the tubing is disconnected from the patient and the tubing clamp is open.
2. Press and hold

You will hear a single beep, and the word Prime and 3 sets of dashes, each accompanied by a beep, will appear on the display.
3. After Prime and 3 sets of dashes appear, release

4. Press and hold

again to fill the fluid path. The screen displays Priming . . . and you will hear a short beep each time the pump goes through a delivery cycle.
NOTE: Medication delivered during priming is subtracted from the reservoir volume, but is not added to the given screen since this amount is not delivered to the patient.
5. If the tubing is not yet fully primed, press and hold

again. If the tubing is primed, press

to return to the main screen.
NOTE: Each time you press and hold

you pump a maximum of 1 ml of medication into the tubing. The pumping action will stop automatically when 1 ml has been delivered. Release

if you finish priming the fluid path sooner. If the fluid path is not fully primed, repeat the above priming procedure.
6. Connect the tubing to the patient’s enteral access device.
7. Set the lock level for the patient (see Setting the Lock Level for the Patient in this section).
Setting the Lock Level for the Patient
The lock level must be changed to LL1 or LL2 to prevent the patient from having complete access to all programming and operating functions.
NOTE: You may change the lock level at any time by stopping the pump and following the procedure below.
To change the lock level
**Text Omitted**
WARNING: Do not disclose to the patient the pump’s security codes or any other information that would allow the patient complete access to all programming and operating functions. Improper programming could compromise patient treatment. Programming with Upper Limits, Adjusting Doses in Lock Level 1
If a prescription allows for the continuous rate, extra dose, or morning dose to be adjusted during the course of therapy, you may wish to operate the pump in LL1. Then, when necessary, you can adjust values up to the maximum value that was programmed in LL0.
Programming the pump to use this feature
- During initial programming in LL0, enter the upper limit values for the continuous rate, extra dose and/or morning dose. (These will be the maximum values when the pump is in LL1.)
- After you are finished programming, change the lock level to LL1.
- Decrease the continuous rate, extra dose and/or morning dose to its starting value, then press

Adjusting the rate or dose while the pump is in use
- If it becomes necessary to increase or decrease the continuous rate, and/or extra dose during the course of therapy, stop the pump but remain in LL1.
- To increase or decrease the morning dose, the pump must be in LL1, but it must remain running.
1. To change the continuous rate or extra dose, press

until the continuous rate or extra dose screen appears.
To change the morning dose, press

2. Press

or

to select the desired value, then press

NOTE: You will not be able to adjust the continuous rate, extra dose, or morning dose beyond the value originally programmed in LL0.
3. Restart the pump if appropriate.
3.0 Operating the Pump
Starting the Pump
When you start the pump, programmed values will be automatically reviewed. Then medication delivery will begin as programmed, and RUN will appear on the main screen. If the pump will not start, a message will appear on the display. Refer to the Messages and Alarms Table in Section 5.
CAUTION: Prior to starting medication delivery, inspect the fluid path for kinks, a closed clamp, or other obstruction. An undetected occlusion may result in under- or non-delivery and/or nuisance alarms. To start the pump
1. Press and hold

Starting and 3 sets of dashes appear on the display; then the dashes disappear one-by-one, each accompanied by a single beep.
2. Release

after the last set of dashes disappears, and the pump beeps. All of the programming screens appear for your review one after the other.
Stopping the Pump
Stopping the pump stops delivery. When the pump is stopped, STOPPED will appear on the main screen, and you will hear 3 beeps every 5 minutes.
To stop the pump
1. Press and hold

Stopping and 3 sets of dashes appear one-by-one on the pump’s display, each accompanied by a single beep.
2. Release

after the third set of dashes appears and the pump beeps.
Turning the Pump On/Off
When the pump is stopped, you may put the pump into a low power state by turning it off. The pump may be turned off when it is disconnected from the patient and it is going to be stored for short periods of time.
CAUTION: Do not store the pump for prolonged periods of time with the batteries installed. Battery leakage could damage the pump. To turn the pump off
1. Press and hold

Turning off and 3 sets of dots will appear one-by-one on the pump’s display, each accompanied by a single beep.
2. Release

The display turns off.
To turn the pump on
1. Press and hold

until the display turns on.
2. Release

The pump will power up and automatically review all screens.
Starting a Morning Dose
A morning dose may be delivered in any lock level while the pump is running. It allows you to deliver a specified amount of medication, as a loading dose for example.
If the patient attempts to deliver a morning dose during the lockout time, the pump will not deliver the dose. The lockout time is determined by the value entered in Morning Dose Lockout, in biomed functions. The extra dose lockout setting has no effect on morning dose frequency. A morning dose may be stopped in progress.
NOTE: A morning dose cannot be started while an extra dose or another morning dose is in progress.
To start a morning dose
To program a morning dose, the pump must be running and a medication cassette reservoir must be attached.
NOTE: The

key must be pressed twice for morning dose delivery to start.
- Make sure the pump is running (in any lock level). Start the pump if necessary.
- Press

The current morning dose value (or the default value of 0 ml) will appear UNLESS the morning dose is currently locked out (in which case the screen will not appear). If the desired morning dose amount appears in the display, press

again to begin delivery.
NOTE: If the desired morning dose amount does not appear in the display, program the desired morning dose amount as instructed in Section 2, Pump Setup and Programming.
The screen will show the value decreasing as the morning dose is delivered.
Starting an Extra Dose
If an extra dose has been programmed, the patient may start an extra dose while the pump is running. The amount delivered is added to the amount provided by the continuous rate.
If the patient attempts to deliver an extra dose during the lockout time, the pump will not deliver the dose. The lockout time is determined by the value entered in Extra Dose Lockout, in biomed functions.
NOTE: An extra dose cannot be started while another extra dose or a morning dose is in progress.
To start an extra dose
- Make sure the pump is running (in any lock level). Start the pump if necessary.
- Press

Two beeps will sound and the pump will begin delivering the extra dose.
As the extra dose is delivered, the main screen will show DOSE in place of RUN.Stopping an Extra Dose or Morning Dose
An extra dose or morning dose can be stopped in progress. The pump may be in any lock level.
To stop an extra dose or morning dose in progress
- Press and hold

to stop the pump. All delivery is stopped, including the continuous rate.
Resetting the Reservoir Volume
NOTE: The default setting for Reservoir Volume is Not in Use. Programming a reservoir volume value is not required for general use, but is available at provider discretion.
To reset the reservoir volume to the value programmed in LL0, the pump may be in any lock level.
1. Stop the pump.
2. Press

to display the reservoir volume screen.
3. Press

to reset the volume to the programmed value.
4.0 Biomed Functions
Overview: Accessing the Biomed Functions and Programming the Lockouts
The biomed functions are pump configurations that are less frequently changed. The biomed functions are accessible only when the pump is stopped and in lock level 0 (LL0).
To access the Biomed Functions
**Text Omitted**
WARNING: Do not disclose to the patient the pump’s security codes or any other information that would allow the patient complete access to all programming and operating functions. Improper programming could compromise patient treatment. 4. Press

to select the setting you wish to view or change, then follow the instructions in this section for the appropriate screen.
NOTE: To leave a biomed function setting unchanged, press

5. To exit the biomed functions, press

until you get to the screen that reads, NEXT for Biomed, ENTER for Main.
6. Press

to return to the main screen.
Extra Dose Lockout
The extra dose lockout time determines how often a patient can receive an extra dose of medication. The lockout time is the minimum amount of time which must elapse between the start of one dose and the start of the next.
To program an extra dose lockout time 1. With the pump stopped and in LL0, access biomed functions. (Refer to the beginning of the Biomed Functions section for instructions on how to access biomed functions.) 2. Press

until Extra Dose appears.
3. Press

or

to scroll to the desired lockout time. The default lockout time is 1 hour.
4. Press

to enter the value.
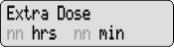
CAUTION: When you enter a new value, any lockout time already in effect will be cleared. An extra dose could be requested and delivered immediately upon starting the pump, which may result in over-delivery of medication. Morning Dose Lockout
The morning dose lockout time determines how often a patient can receive a morning dose. The lockout time is the minimum amount of time which must elapse between the start of one dose and the start of the next.
To program a morning dose lockout time 1. With the pump stopped and in LL0, access biomed functions. (Refer to the beginning of the Biomed Functions section for instructions on how to access biomed functions.) 2. Press

until Morning Dose appears.
3. Press

or

to scroll to the desired lockout time.
4. Press

to enter the value.
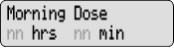
CAUTION: When you enter a new value, any lockout time already in effect will be cleared. A morning dose could be requested and delivered immediately upon starting the pump, which may result in over-delivery of medication. Upstream Occlusion Sensor On/Off
The upstream occlusion sensor can be set to On or Off. If this screen is set to On, and an occlusion in the medication cassette reservoir is detected, an alarm will sound, delivery will stop, and the display will show Upstream Occlusion.
CAUTION: When the upstream occlusion sensor is turned off, the pump will not detect occlusions in the medication cassette reservoir. Periodically inspect the medication cassette reservoir for decreasing volume. Undetected occlusions could result in under- or non-delivery of medication. 1. With the pump stopped and in LL0, access biomed functions. (Refer to the beginning of the Biomed Functions section for instructions on how to access biomed functions.) 2. Press

until Upstream Sensor appears.
3. Press

or

to select Off or On.
4. Press

to enter the change.
5.0 Reference
Messages and Alarms, Alphabetical List
Messages and Alarms Description / Corrective Action [No message]
Two-tone alarmWith no AC adapter attached, the batteries have been removed while the pump is running. The pump is now stopped and unpowered. Install batteries to silence the alarm.
OR
Batteries were removed within approximately 15 seconds after stopping the pump. Install new batteries to silence the alarm, if desired. Otherwise, the alarm will stop within a short period of time.[Screen displays current pump status]
Two-beeps (long-short)The medication cassette reservoir is not aligned with the pump or medication is not flowing from the medication bag inside the reservoir to the pumping mechanism.
Press

or

to silence the alarm. The pump continues to run. Make sure the medication cassette reservoir is properly aligned with the pump and medication is flowing from the medication bag to the pumping mechanism. Very cold or extremely thick medication may cause this alarm as well. Allow the medication cassette reservoir to thaw to room temperature before attaching to the pump.Battery Depleted
Two-tone alarmThe battery power is too low to operate the pump. The pump is now stopped.
- Change the batteries immediately.
- Press and hold

to restart the pump.Battery Removed
Pump won’t run
Two-tone alarmWith the AC adapter attached, the AA batteries have been removed while the pump is running, or you have tried to start the pump with depleted batteries. The pump is now stopped. Press

or

to silence the alarm. Reinstall batteries or install new batteries. Press and hold

to restart the pump.Error
Two-tone alarmAn error has occurred. Remove the pump from service and contact Smiths Medical to return the pump for service. High Pressure
Two-tone alarmThe pump has detected high pressure, which may be resulting from a downstream blockage, kink in the fluid path, or a closed clamp. Remove the occlusion to resume operation. Or, press

or

to stop the pump and silence the alarm for 2 minutes, then remove the occlusion and restart the pump.Key pressed,
Please release
Two-tone alarmIf a key is being pressed, stop pressing it. If the alarm persists, close the tubing clamp and remove the pump from use. Contact Smiths Medical to return the pump for service. LowBat
Three two-tone beeps every 5 minutesThe batteries are low, but the pump is still operable. - Change the batteries soon.
Motor Locked, remove all power
Two-tone alarmBatteries are depleted and the pump was powered up with the AC adapter. Install new AA batteries, reconnect the AC adapter, and restart the pump. No Disposable,
Clamp Tubing
Two-tone alarmThe disposable (medication cassette reservoir) was removed. The pump is not sensing proper cassette attachment. Clamp the tubing and disconnect from the enteral access device. A medication cassette reservoir must be properly attached in order for the pump to run. Press

or

to silence the alarm.No Disposable,
Pump won’t run
Two-tone alarmYou have tried to start the pump without a disposable (medication cassette reservoir) attached. A medication cassette reservoir must be properly attached in order for the pump to run. Press

or

to silence the alarm.Power lost while pump was on
Two-tone alarmThe pump was running when power was removed. Stop the pump before changing the batteries or removing the power source. Press

or

to silence the alarm.Programming Incomplete
Two-tone alarm when starting the pumpA rate or dose must be programmed to start the pump. Press

or

to silence the alarm.Reservoir Volume Empty
Two-tone alarmThe reservoir volume has reached 0.0 ml. Press

or

to silence the alarm. Then install a new medication cassette reservoir if appropriate and reset the reservoir volume.RUN
ResVol Low
Three single beepsThe reservoir volume is low. Change the medication cassette reservoir soon. See Reservoir Volume Alarm in Section 1 for further details. Service Due
Two-tone alarmThe pump is functional, but is due for service based on clock battery age or total motor revolutions. This screen appears in LL0 for 60 days and then in all lock levels until returned for service. Upstream Occlusion
Two-tone alarmIf the upstream occlusion sensor is set to On and an occlusion in the medication cassette reservoir is detected, the upstream occlusion alarm will sound. Press

or

to stop the pump and silence the alarm for 2 minutes, then remove the occlusion and restart the pump. You may have to detach the cassette from the pump, then reattach it.Value not saved A value was not saved by pressing

Press

to resume programming. Verify all programming screens before moving to the next screen or starting the pump.What if the pump is dropped or hit? Immediately do the following: - Check the cassette latch on the side of the pump and make sure the line on the latch lines up with the arrow on the side of the pump.
- Gently twist, push, and pull on the medication cassette reservoir to make sure it is still firmly attached.
- Check the battery door to make sure it is still firmly attached.
If the medication cassette reservoir or the battery door is loose or damaged, do not use the pump. Immediately stop the pump, close the tubing clamp, and contact Smiths Medical.
WARNING: If the pump is dropped or hit, inspect the pump for damage. Do not use a pump that is damaged or is not functioning properly, as this could compromise patient treatment. - If the pump is accidentally dropped in water, retrieve it quickly, dry it off with a towel, and contact Smiths Medical.
Cleaning the Pump and Accessories
CAUTION: -
Do not immerse the pump in cleaning fluid or water. Do not allow solution to soak into the pump, accumulate on the keypad, or enter the battery compartment. Moisture buildup inside the pump may damage the pump.
- Do not clean the pump with acetone, other plastic solvents, or abrasive cleaners, as damage to the pump may occur.
NOTE: Refer to the Instructions for Use for each accessory before proceeding with cleaning.
The following solutions may be used to clean the pump and accessories, unless otherwise specified:
- Soap solution
- Benzalkonium chloride concentrate (0.13%)
- Glutaral concentrate, USP (2%)
- 10% solution of household bleach (one part household bleach to 9 parts water)
- Alcohol, USP (93%)
- Isopropyl Alcohol, USP (99%)
- Chlorhexidine gluconate (4%)
- PDI Super Sani-Cloth®
- MadaCide, Mada Medical
- Dampen a soft, lint-free cloth with cleaning solution. Apply the solution to the exterior surface of the pump (per manufacturer’s instructions). Do not allow the solution to soak into the pump or accessory.
- Wipe the entire surface dry with another soft, lint-free cloth. Allow the pump to dry completely before use.
Cleaning the Battery Contacts
Routinely clean the battery contacts, possibly as part of the preventative maintenance cycle, to remove buildup of foreign material on the contacts.
Use the following to clean the battery contacts:
- Cotton swab wetted with Isopropyl Alcohol (70% minimum)
NOTE: Do not use an alcohol formulation that contains components other than alcohol and water.
OR
- Pre-moistened alcohol swab
- Using a swab wetted with alcohol, rub the entire battery contact for a minimum of 10 back and forth cycles (20 total wipes over the contact).
- Using a clean surface of the swab, repeat process for second battery contact.
- Using a clean swab wetted with alcohol, rub each battery contact again, a minimum of 4 back and forth cycles (8 total wipes over the contact).
- Allow the contacts to dry completely before use.
Exposure to Radiation, Ultrasound, Magnetic Resonance Imaging (MRI), or Use near ECG Equipment
CAUTION: -
Do not expose the pump to therapeutic levels of ionizing radiation as permanent damage to the pump’s electronic circuitry may occur. The best procedure to follow is to remove the pump from the patient during therapeutic radiation sessions. If the pump must remain in the vicinity during a therapy session, it should be shielded, and its ability to function properly should be confirmed following treatment.
-
Do not expose the pump directly to ultrasound, as permanent damage to the electronic circuitry may occur.
-
Do not use the pump in the vicinity of magnetic resonance imaging (MRI) equipment as magnetic fields may adversely affect the operation of the pump. Remove the pump from the patient during MRI procedures and keep it at a safe distance from magnetic energy.
- This pump may interfere with ECG equipment. Monitor ECG equipment carefully when using this pump.
Continuous Rate Scroll Ranges
Starting Value Increment Maximum 0.0 ml/hr 0.1 ml/hr 20.0 ml/hr Extra Dose, Morning Dose Scroll Ranges
Extra Dose increment max. 0.1 ml 9.9 ml Morning Dose increment max. 0.1 ml 20 ml Technical Description
Standards used in Development of the Pump The following standards were used in whole or part in the development of the pump: IEC 60601-1 (1988), (2nd Edition, 1988) Medical Electrical Equipment, Part 1: General Requirements for Safety. Amendment 1 (1991), Amendment 2 (1995). EN 60601-1 (1990), Medical Electrical Equipment, Part 1: General Requirements for Safety. Amendment A1 (1993), Amendment A13 (1996), Amendment A2 (1995). IEC 60601-1-2 (2007), Medical Electrical Equipment, Part 1-2: General Requirements for Safety – Collateral Standard: Electromagnetic Compatibility – Requirements and Tests. EN 60601-1-2 (2007), Medical Electrical Equipment, Part 1-2: General Requirements for Safety – Collateral Standard: Electromagnetic Compatibility – Requirements and Tests. The following are reference test methods applied to IEC/EN 60601-1-2: IEC/EN 61000-4-2, Electromagnetic Compatibility (EMC), Part 4: Testing and measurement techniques. Section 2: Electrostatic Discharge immunity test. Basic EMC Publication. IEC/EN 61000-4-3, Electromagnetic Compatibility (EMC), Part 4: Testing and measurement techniques. Section 3: Radiated, radio frequency, electromagnetic fields immunity test. Basic EMC Publication. IEC/EN 61000-4-4, Electromagnetic Compatibility (EMC), Part 4: Testing and measurement techniques. Section 4: Electrical fast transients/bursts immunity test. Basic EMC Publication. IEC/EN 61000-4-5, Electromagnetic Compatibility (EMC), Part 4: Testing and measurement techniques. Section 5: Surge immunity test. Basic EMC Publication. IEC/EN 61000-4-8, Electromagnetic Compatibility (EMC), Part 4: Testing and measurement techniques. Section 8: Power frequency magnetic field immunity test. IEC/EN 61000-4-11, Electromagnetic compatibility (EMC) - Part 4: Testing and measuring techniques - Section 11: Voltage dips, short interruptions and voltage variations immunity tests. IEC 60601-2-24 (1998), Medical Electrical Equipment, Part 2-24: Particular Requirements for Safety of Infusion Pumps and Controllers. EN 60601-2-24 (1998), Medical Electrical Equipment, Part 2-24: Particular Requirements for Safety of Infusion Pumps and Controllers. IEC 60601-1-4 (2000), Medical Electrical Equipment, Part 1: General Requirements for Safety – Collateral Standard: Programmable electrical medical systems. EN 60601-1-4 (1996), Medical Electrical Equipment, Part 1-4: General Requirements for Safety – Collateral Standard: Programmable electrical medical systems. Amendment A1: 1999. EN 980 (2008), Graphical symbols for use in the labeling of medical devices. FCC Part 15 Subpart B, Radiofrequency Devices, Unintentional Radiators. RTCA/DO -160C, Radiated Emissions Only, Category A & Z Limit. EN 55011 (2007), Limits and Methods of Measurement of Radio Interference Characteristics of Industrial, Scientific and Medical (ISM) Equipment. Amendment A2: 2007. (Equivalent to CISPR 11: 2003 + Amendment A2: 2006). CISPR11 (2009), Limits and methods of measurement of electromagnetic disturbance characteristics of industrial, scientific and medical (ISM) radio frequency equipment. Amendment 1 (1996) Amendment 2 (1996). For CISPR11 tests, the pump was fitted with an administration set with its inlet connected to a 250 ml bag and its outlet routed back to the bag forming a closed loop system. A total of 6 feet of tubing was used to form the closed loop. Specifications (Nominal)
General Pump Specifications Resolution medication cassette reservoir: 0.05 ml per pump activation nominal Size 4.1 cm × 9.5 cm × 11.2 cm [1.6 in. × 3.8 in. × 4.4 in.] excluding medication cassette reservoir Weight 392 g [13.8 oz.] including 2 AA batteries, empty 100 ml medication cassette reservoir, excluding other accessories Classification (IEC 60601-1) CF

Class II

Moisture protection Splashproof (IPX4) Pump alarms Low battery power; depleted battery power; battery dislodged; pump stopped; pump fault; low reservoir volume; high pressure; disposable not attached when run attempted; motor locked; upstream occlusion; reservoir volume empty; program incomplete; remote dose cord removed; key stuck; disposable detached. Maximum infusion pressure 40 psi [2.76 bar] Maximum time to occlusion alarm (actual test data) DUOPA (carbidopa and levodopa) enteral suspension: 10 hours 30 minutes Bolus volume at occlusion alarm pressure 0.05 ml resolution medication cassette reservoir: <1.1 ml Power sources AC adapter; Two AA alkaline batteries Battery life The expected life of 2 AA batteries is 100 hours at 100 ml/16 hours, or approximately 14 days at 10 ml/day (nominal). This estimate is based on laboratory tests conducted at room temperature using two new batteries. Actual battery life will vary depending on the brand of battery, battery shelf life, temperature conditions, and delivery rate. It is recommended that two new AA batteries be kept available for replacement if necessary.
An internal battery powers the clock. When it is depleted, it cannot reliably maintain the clock time. This battery must be replaced by the manufacturer. The internal battery has an expected life of 5 years.System operating temperature 2°C to 40°C (36°F to 104°F) System storage and transportation temperature -20°C to 60°C (-4°F to 140°F) When shipping, use pump case. System delivery accuracy (DUOPA [carbidopa and levodopa] enteral suspension): Therapy1 ±10% Continuous rate2 ±10% @ flow rates 0.4 ml/hr to 20 ml/hr Extra dose2 ±10% Morning dose2,3 ±10% Accessories: 100 ml medication cassette reservoir with 36” 0.10” I.D. tube 1Therapy delivery accuracy: program to deliver a 10 ml morning dose with a 5 ml/hr continuous rate and three 3 ml extra doses, with a total of 100 ml of medication delivered. Pumps were evaluated in latch down, upside down, and horizontal position. The medication cassette with DUOPA (carbidopa and levodopa) enteral suspension is initially cold (approx. 2°C). Remove cassette from refrigerator, wait 20 minutes, then conduct the test at nominal temperature.2
2At nominal temperature (23°C ± 5°C)
3An additional -4% change may be seen when cassette starting temperature is 2°C and placed at room temperature (23°C ± 5°C) for 20 minutes prior to use.WARNING: System delivery inaccuracies beyond the stated accuracy may occur as a result of back pressure or fluid resistance, which depends upon temperature, medication viscosity, catheter size, extension set tubing, flow rate, and orientation of the pump system. System definition System is defined as a CADD-Legacy® 1400 pump with attached medication cassette reservoir supplied by AbbVie High pressure alarm 26 ± 14 psi [1.79 ± 0.97 bar] Maximum volume infused under single fault condition medication cassette reservoir: 0.2 ml. Delivery rate during priming Approx. 125 ml/hr Delivery Specifications Reservoir volume 1 to 9999 or Not In Use; programmable in 1 ml increments, displayed in 0.1 ml increments Default: Not In Use Continuous rate 0 to 20 ml/hr in 0.1 ml/hr increments
Default: 0.1 ml/hrExtra dose 0 to 9.9 ml in 0.1 ml increments
Delivery rate (continuous rate + extra dose): 40 ml/hr
Default: 0 mlGiven 0 to 99999.95 in 0.05 unit increments Morning dose 0 to 20.00 ml in 0.1 ml increments
Delivery rate (continuous rate + morning dose):
40 ml/hr
Default: 0 mlBiomed Functions Extra dose lockout 15 minutes to 24 hours in 15 minute increments
Default: 1 hourMorning dose lockout 1 to 24 hours in 1 hour increments Default: 20 hours Upstream sensor Off
On
Default: OffAccuracy Test Results
The following graphs are designed to show flow accuracy of the infusion system plotted against given time periods. The medication cassette reservoir used for flow accuracy tests was supplied by AbbVie.
Flow rate immediately following startup Time Interval: 0.5 min Total Time: 120 min Programmed Rate: 5 ml/hr Reservoir Used: 100 ml medication cassette reservoir 
Short term flow rate error Programmed Rate: 5 ml/hr Average Flow Rate: 4.76 ml/hr Mean Flow Error: –4.65% Reservoir Used: 100 ml medication cassette reservoir 
Electromagnetic Emissions and Immunity Declarations
Electromagnetic emissions declaration The Pump is intended for use in the electromagnetic environment specified below. The customer or the user of the Pump should assure that it is used in such an environment. Emissions test Compliance Electromagnetic environment – guidance RF emissions, CISPR 11 Group 1 The Pump uses RF energy only for its internal function. Therefore, its RF emissions are very low and are not likely to cause any interference in nearby electronic equipment. RF emissions, CISPR 11 Class B The Pump is suitable for use in all establishments, including domestic establishments and those directly connected to the public low-voltage power supply network that supplies buildings used for domestic purposes. Harmonic emissions
IEC 61000-3-2Not applicable Voltage fluctuations/
flicker emissions
IEC 61000-3-3Not applicable Compliance using:
- 115VAC/60HZ to 8VDC Power Adapter (US) with a cord length of 274 ± 10 cm (108 ± 4 in).
- Remote Dose Cord with a length of 152 ± 5 cm (60 ± 2 in).
WARNING: -
The Pump should not be used adjacent to or stacked with other equipment. If adjacent or stacked use is necessary, the user should verify normal operation of the pump in the configuration in which it is to be used.
- The use of power supplies and a remote dose cord other than those listed in the electromagnetic emissions declaration may result in increased emissions or decreased immunity of the Pump.
Electromagnetic immunity declaration The Pump is intended for use in the electromagnetic environment specified below. The customer or the user of the Pump should assure that it is used in such an environment. Immunity test IEC 60601
test levelCompliance
levelElectromagnetic environment – guidance Electrostatic discharge (ESD)
IEC 61000-4-2±8 kV contact
±15 kV air±8 kV contact
±15 kV airFloors should be wood, concrete or ceramic tile. If floors are covered with synthetic material, the relative humidity should be at least 30%. Electrical fast transient/burst
IEC 61000-4-4±2 kV for power supply lines
±1 kV for input/output lines±2 kV for power supply lines
Not applicableMains power quality should be that of a typical commercial or hospital environment. Surge
IEC 61000-4-5±1 kV differential mode
±2 kV common mode±1 kV differential mode
±2 kV common modeMains power quality should be that of a typical commercial or hospital environment. Voltage dips, short interruptions and voltage variations on power supply input lines
IEC 61000-4-11<5 % UT (>95 % dip in UT) for 0,5 cycle
40 % UT (60 % dip in UT) for 5 cycles
70 % UT (30 % dip in UT) for 25 cycles
<5 % UT (>95 % dip in UT) for 5 sec<5 % UT (>95 % dip in UT) for 0,5 cycle
40 % UT (60 % dip in UT) for 5 cycles
70 % UT (30 % dip in UT) for 25 cycles
<5 % UT (>95 % dip in UT) for 5 secMains power quality should be that of a typical commercial or hospital environment. If the user of the Pump requires continued operation during power mains interruptions, it is recommended that the Pump be powered from an uninterruptible power supply or a battery. Power frequency 50/60 Hz) magnetic field
IEC 61000-4-83 A/m 400 A/m
(IEC 60601-2-24)Power frequency magnetic fields should be at levels characteristic of a typical location in a typical commercial or hospital environment. NOTE: UT is the a.c. mains voltage prior to application of the test level. Electromagnetic immunity declaration The Pump is intended for use in the electromagnetic environment specified below. The customer or the user of the Pump should assure that it is used in such an environment. Immunity test IEC 60601
test levelCompliance
levelElectromagnetic environment – guidance Portable and mobile RF communications equipment should be used no closer to any part of the Pump, including cables, than the recommended separation distance calculated from the equation applicable to the frequency of the transmitter. Conducted RF
IEC 61000-4-63 Vrms
150 kHz to
80 MHz10 Vrms Recommended separation distance
d=0.27*P1/2Radiated RF
IEC 61000-4-33 V/m
80 MHz to
2,5 GHz10 V/m d=0.27*P1/2 80MHz to 800 MHz
d=0.54*P1/2 800MHz to 2,5 GHz
Where P is the maximum output power rating of the transmitter in watts (W) according to the transmitter manufacturer and d is the recommended separation distance in meters (m).
Field strengths from fixed RF transmitters, as determined by an electromagnetic site survey,a should be less than the compliance level in each frequency range.b
Interference may occur in the vicinity of equipment marked with the following symbol:
NOTE 1: At 80 MHz and 800 MHz, the higher frequency range applies. NOTE 2: These guidelines may not apply in all situations. Electromagnetic propagation is affected by absorption and reflection from structures, objects and people. aField strengths from fixed transmitters, such as base stations for radio (cellular/cordless) telephones and land mobile radios, amateur radio, AM and FM radio broadcast and TV broadcast cannot be predicted theoretically with accuracy. To assess the electromagnetic environment due to fixed RF transmitters, an electromagnetic site survey should be considered. If the measured field strength in the location in which the Pump is used exceeds the applicable RF compliance level above, the Pump should be observed to verify normal operation. If abnormal performance is observed, additional measures may be necessary, such as reorienting or relocating the Pump. bOver the frequency range 150 kHz to 80 MHz, field strengths should be less than 10 V/m. Recommended separation distances between portable and mobile RF communications equipment and the Pump The Pump is intended for use in an electromagnetic environment in which radiated RF disturbances are controlled. The customer or the user of the Pump can help prevent electromagnetic interference by maintaining a minimum distance between portable and mobile RF communications equipment (transmitters) and the Pump as recommended below, according to the maximum output power of the communications equipment. Rated maximum output power of transmitter Separation distance according to frequency of transmitter
mW 150 kHz to 80 MHz
d=0.27*P1/280 MHz to 800 MHz
d=0.27*P1/2800 MHz to 2,5 GHz
d=0.54*P1/20.01 0.03 0.03 0.05 0.1 0.09 0.09 0.17 1 0.27 0.27 0.54 10 0.85 0.85 1.7 100 2.7 2.7 5.4 For transmitters rated at a maximum output power not listed above, the recommended separation distance d in meters (m) can be determined using the equation applicable to the frequency of the transmitter, where P is the maximum output power rating of the transmitter in watts (W) according to the transmitter manufacturer. NOTE 1: At 80 MHz and 800 MHz, the separation distance for the higher frequency range applies. NOTE 2: These guidelines may not apply in all situations. Electromagnetic propagation is affected by absorption and reflection from structures, objects and people. Safety Features and Fault Detection
Hardware Safety Features
Key hardware safety features include a watchdog timer circuit, motor driver and motor watchdog circuits, and a voltage detector circuit. Each safety circuit performs a unique function to insure the overall safety of the device.
Watchdog Timer Circuit
The microprocessor must send an appropriate signal to the watchdog circuit at least once per second. If the microprocessor does not, the watchdog circuit will time out and shut down the pump controller.
Watchdog timer circuitry is provided to monitor the status of the microprocessor and disable the motor and enable the audible alarm if the microprocessor fails to function properly. The microprocessor must strobe the watchdog circuit at least once every second in order to prevent the watchdog from performing its reset function. The reset output from the watchdog circuit is a pulse output. This acts to “jump startˮ the microprocessor. This unique feature allows the microprocessor to test the watchdog circuit on every power-up.
By setting a flag in the memory and not strobing the watchdog, the microprocessor can force a watchdog time-out. After being reset, the microprocessor checks the status flag to see if this was a time-out test. If so, the microprocessor continues normal power-up activities. If the reset occurred when the microprocessor was not expecting it, the microprocessor traps the event, sounds the audible alarm and displays an error message on the LCD.
Motor Driver/Motor Watchdog Circuit
Motor drive circuitry is composed of a series of power FET transistors, passive components, and 2 voltage comparators. Built into the motor drive circuitry is an RC timer which times how long the motor runs each time it is turned on. If the motor runs for more than an average of 3 seconds, the circuit will time out and disable the motor. A unique feature of this circuit is that control lines to and from the microprocessor circuit allow the microprocessor to perform a complete functional test of the motor drive circuit without running the motor. The microprocessor performs this test function every several minutes to assure its continued functionality. An input from the watchdog circuit prevents motor operation if the watchdog timer expires. The software verifies this function during the watchdog test described above.
Voltage Detector Circuit
Low voltage detection is performed by part of the watchdog circuit and by the microprocessor via software. Three low voltage levels are detected. The first 2 levels are detected by software and the third by hardware. The first level to be reached is the low battery warning threshold which occurs when the battery voltage decays to a nominal value of 2.4 volts when the motor is off or 1.8 volts when the motor is active. An analog to digital converter (ADC) built into the microprocessor allows the microprocessor, via software, to monitor the battery voltage. At the low battery warning threshold, the microprocessor enables a periodic series of beeps and displays a low battery warning message on the LCD. As the voltage operating the motor reaches a nominal value of 4.75 volts, the software disables delivery, places a battery depleted message on the LCD, and enables a constant two tone audible alarm. When the battery voltage decays to a nominal value of 1.0 volts, a hardware reset circuit is triggered which places the microprocessor in reset. This prevents ambiguous microprocessor operation when the battery voltage continues to decay. The hardware reset continues until the battery is completely discharged or until it is removed. Once the pump controller goes into low battery shutdown, only replacing the depleted batteries with new ones will clear the condition.
Software Safety Features
Hardware-related Software Safety Features
Program Memory Check
At power up and at regular intervals thereafter, the program memory is tested by calculating a cyclic redundancy code (CRC) on the program and then comparing it with the CRC stored with the program.
If the stored and calculated CRCs do not match, the software will display a system fault screen, turn on a continuous two-tone audible alarm, and stop all medication delivery.
RAM Memory Check
At power up, the random access memory is checked. A series of bit patterns is written to and read from each address in the RAM. If the read data is different from the written data, the software will display a system fault screen, turn on a continuous two-tone audible alarm, and stop all medication delivery.
Motor Circuit Check
At power up and at regular intervals thereafter, the motor circuit is checked to ensure that no power is being applied to the motor unless the motor is actually on. If the software detects power being applied to the motor at any other time, it will sound a continuous two-tone audible alarm and will no longer attempt to deliver medication. During every pump activation, the software checks to see whether the motor completes one activation. If the motor fails to turn, or fails to complete a cycle, the software will display a system fault screen, turn on a continuous two-tone audible alarm, and stop all medication delivery.
Keyboard Encoder Check
Every time the software receives data from the keyboard encoder, it is checked. If the data is not a valid key press, the software will disregard the key press. The keyboard is designed with redundant switches for

,

, and

. The software must detect that both switches are activated before taking any action.
Data Handling Software Safety Features
Data Stored in RAM
Before use, data associated with delivery and stored in RAM is tested by calculating a CRC on the data and then comparing it with the CRC stored with the data. If the stored and calculated CRCs do not match, the software will display a system fault screen, turn on a continuous two-tone audible alarm, and stop all medication delivery.
Data Stored in EEPROM
Before use, data associated with delivery and stored in EEPROM is tested by calculating a CRC on the data and then comparing it with the CRC stored with the data. If the stored and calculated CRCs do not match, the software will display a system fault screen, turn on a continuous two-tone audible alarm, and stop all medication delivery.
Data Stored in NOVRAM
Before use, data associated with delivery and stored in NOVRAM is tested by calculating a CRC on the data and then comparing it with the CRC stored with the data. If the stored and calculated CRCs do not match, the software will display a system fault screen, turn on a continuous two-tone audible alarm, and stop all medication delivery.
Data Used in Calculations
Calculations on data used in some way to control the delivery of medication are performed redundantly.
The two calculated values are then compared. If the two values do not match, the software will display a system fault screen, turn on a continuous two-tone audible alarm, and stop all medication delivery.
Timer Data Registers
The data in the real time clock is checked at regular intervals. If the data is not reasonable, the software will turn on a continuous two-tone audible alarm and stop all medication delivery.
Annual Functional Inspection
Smiths Medical recommends annual functional inspections and tests on the CADD-Legacy® 1400 pump. Contact Smiths Medical to coordinate return and inspection of the pump.
CAUTION: CADD-Legacy® 1400 pumps are sealed units. A broken or damaged seal will, therefore, be considered conclusive evidence that the pump has been misused and/or altered, which voids any and all warranties. All service and repair of CADD-Legacy® 1400 pumps must be performed by Smiths Medical or its authorized agents. Collect Separately
This product contains electronic and other components (such as batteries) that may contain materials which, if disposed of with general household waste, could be damaging to the environment.
In accordance with Directive 2002/96/EC Waste Electrical and Electronic Equipment, Smiths Medical requires that residents of the European Union return this product for proper disposal at the end of its useful life.
If you are unsure of the proper disposal method, contact your local distributor for specific disposal instructions.
WARNING: There are potential health hazards associated with improper disposal of batteries, electronics, and contaminated (used) reservoirs and extension sets. Dispose of used batteries, reservoirs, extension sets and other used accessories, or a pump that has reached the end of its useful life, in an environmentally safe manner, and according to any regulations that may apply. Limited Warranty
Smiths Medical ASD, Inc. (the “Manufacturer”) warrants to the Original Purchaser that the infusion pump (the “Pump”), not including accessories, shall be free from defects in materials and workmanship under normal use, if used in accordance with this Operator’s Manual, for a period of one year from the actual date of sale to the Original Purchaser. THERE ARE NO OTHER WARRANTIES.
This warranty does not cover normal wear and tear and maintenance items, and specifically excludes batteries, administration sets, extension sets or any other accessory items or equipment used with the Pump.
Subject to the conditions of and upon compliance with this Limited Warranty, the Manufacturer will repair or replace at its option without charge (except for a minimal charge for postage and handling) any Pump (not including accessories) which is defective if a claim is made during such one-year period.
The following conditions, procedures, and limitations apply to the Manufacturer’s obligation under this warranty:
A. Parties Covered by this Warranty: This warranty extends only to the Original Purchaser of the Pump. This warranty does not extend to subsequent purchasers. The Original Purchaser may be a patient, medical personnel, a hospital, or institution which purchases the Pump for treatment of patients. The Original Purchaser should retain the invoice or sales receipt as proof as to the actual date of purchase.
B. Warranty Performance Procedure: Notice of the claimed defect must be made in writing or by telephone to the Manufacturer as follows: Smiths Medical ASD, Inc. 1265 Grey Fox Road, St. Paul MN 55112 USA, 1 800.258.5361 (USA). Notice to the Manufacturer must include date of purchase, model and serial number, and a description of the claimed defect in sufficient detail to allow the Manufacturer to determine and facilitate any repairs which may be necessary. AUTHORIZATION MUST BE OBTAINED PRIOR TO RETURNING THE PUMP. If authorized, the Pump must be properly and carefully packaged and returned to the Manufacturer, postage prepaid. Any loss or damage during shipment is at the risk of the sender.
C. Conditions of Warranty: The warranty is void if the Pump has been 1) repaired by someone other than the Manufacturer or its authorized agent; 2) altered so that its stability or reliability is affected; 3) misused; or, 4) damaged by negligence or accident. Misuse includes, but is not limited to, use not in compliance with the Operator’s Manual or use with nonapproved accessories. The Pump is a sealed unit, and the fact that the seal has been broken will be considered conclusive evidence that the Pump has been altered or misused. Removal or damage to the Pump’s serial number will invalidate this warranty.
D. Limitations and Exclusions: Repair or replacement of the Pump or any component part thereof is the EXCLUSIVE remedy offered by the Manufacturer. The following exclusions and limitations shall apply:
- No agent, representative, or employee of the Manufacturer has authority to bind the Manufacturer to any representation or warranty, expressed or implied.
- THERE IS NO WARRANTY OF MERCHANTABILITY OR FITNESS OR USE OF THE PUMP FOR ANY PARTICULAR PURPOSE.
- The Pump can only be used under the supervision of medical personnel whose skill and judgment determine the suitability of the Pump for any particular medical treatment.
- All recommendations, information, and descriptive literature supplied by the Manufacturer or its agents are believed to be accurate and reliable, but do not constitute warranties.
E. Computer Program License:
- The Pump is intended to be used in conjunction with a particular Licensed Computer Program supplied by Manufacturer and use of any other program or unauthorized modification of a Licensed Computer Program shall void Manufacturer’s warranty as set forth above.
- The Original Purchaser and any users authorized by the Original Purchaser are hereby granted a nonexclusive, nontransferable license to use the Licensed Computer Program only in conjunction with the single Pump supplied by Manufacturer. The Licensed Computer Program is supplied only in machine-readable object code form and is based upon Manufacturer’s proprietary confidential information. No rights are granted under this license or otherwise to decompile, produce humanly readable copies of, reverse engineer, modify or create any derivative works based upon the Licensed Computer Program.
- All other terms and conditions of this Limited Warranty shall apply to the Licensed Computer Program.
The Manufacturer disclaims responsibility for the suitability of the Pump for any particular medical treatment or for any medical complications resulting from the use of the Pump. The Manufacturer shall not be responsible for any incidental damages or consequential damages to property, loss of profits, or loss of use caused by any defect or malfunction of the Pump.
This warranty gives the Original Purchaser specific legal rights, and the Original Purchaser may have other legal rights which may vary from state to state.
Index
A AC adapter accessory jack accuracy tests AC indicator light arrow keys B batteries, AA installing battery compartment battery contacts, cleaning battery, internal clock battery life biomed functions extra dose lockout morning dose lockout specifications upstream occlusion sensor on/off biomed functions code C cassette latch cleaning pump continuous rate programming D downstream occlusion sensor E ECG equipment, interference with electromagnetic emissions and immunity declarations extra dose Lockout programming starting stopping extra dose key G given clearing K keypad, keys L latch, cassette lock level changing lock level code M magnetic resonance imaging main screen medication cassette reservoir attaching removing morning dose Lockout starting stopping P power jack power-up priming programming programming, general programming screens R radiation, exposure to reservoir volume programming resetting S safety features hardware software security codes biomed functions code lock level code service due software version specifications biomed functions delivery general standards starting pump stopping pump symbols system definition T turning pump on/off U ultrasound, vii upstream occlusion sensor W warnings and cautions warranty Appendix A – Pump Programming Quick Reference for Healthcare Providers
This quick reference provides for step-by-step directions for several of the common pump programming tasks performed with the CADD-Legacy® 1400 pump. Additional pump information including warnings, cautions and more information on pump operations is located in the referenced sections of the pump Operator’s Manual. Please refer to the full prescribing information for DUOPA (carbidopa and levodopa) enteral suspension for indications and usage, contraindications, warnings, precautions, and adverse reactions.
Begin programming the pump by:
- Attaching a medication cassette reservoir
- Turning on the pump
For instruction on attaching a medication cassette reservoir, see Section 2, Pump Setup and Programming.
You will need the following items to complete these steps:
- Pump
- Medication cassette reservoir
Changing to Lock Level 0 (LL0)
Lock level 0 (LL0) allows the health care provider to adjust settings so they are appropriate for the patient. For more information on lock levels descriptions, see Section 1, General Description. For more information on changing the lock level, see Section 2, Pump Setup and Programming.
**Text Omitted**
Pump Programming Settings
Program the pump settings to customize the medication delivery inputs for the patient. For more information see Section 2, Pump Setup and Programming.
NOTE: Ensure that the pump is in lock level 0 (LL0) and
appears on the screen.
1. Press

Check for

on the screen.
NOTE: Not in Use is the default setting for the reservoir volume. The reservoir volume feature is not required for use, but is available at provider discretion.
2. Press

again. Check for

on the screen.
3. Press

or

to select the desired continuous rate.
4. Press

Pump Status: The continuous rate is now set.
5. Press

Check for
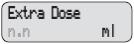
on the screen.
6. Press

or

to select the extra dose amount.
7. Press

Pump Status: The extra dose amount is now set.
8. Press

check for

on the screen
9. Press

if you wish to clear the amount given.
10. Press

NOTE: To change a setting again, press NEXT until the appropriate screen appears. Press

or

to adjust the setting, then press

to confirm.
Changing Lockout Times
Program the dose lockout times to customize medication delivery inputs for each patient. Lockout times will determine how often a patient can deliver a morning dose and an extra dose. These values should be determined during titration. For more information on DUOPA titration, refer to the full prescribing information for DUOPA (carbidopa and levodopa) enteral suspension.
The biomed functions allow the health care provider access to the extra dose lockout and morning dose lockout settings. For more information about biomed functions, see Section 4, Biomed Functions.
NOTE:
- Ensure that the pump is in lock level 0 (LL0) and

is on the screen.
- When entering a new lockout value, any lockout time previously in effect will be cleared.
**Text Omitted**
Programming the Morning Dose
Program the morning dose to customize medication delivery for the patient. For more information, see Section 2, Pump Setup and Programming.
CAUTION: Review programming screens when complete to make sure desired programming has been entered. Check to make sure unintended changes were not made to the morning dose, continuous rate, or extra dose volume. If unintended changes were made, go to the appropriate screen and program the desired value. 1. The pump must be running with a cassette attached and in LL0 or LL1. Start the pump, if necessary and confirm the lock level settings.
NOTE: In LL0, programming in the full range is possible. In LL1, you can program up to the LL0 value.
2. Press


should appear on the screen.
3. Press

or

to select the desired morning dose volume.
4. Press

to store the morning dose volume.
Setting the Lock Level
For patient use, the pump must be set to lock level 2 (LL2) or lock level 1 (LL1). For more information on lock levels descriptions, see Section 1, General Description. For more information on changing the lock level, see Section 2, Pump Setup and Programming.
**Text Omitted**
Manufacturer:
Smiths Medical ASD, Inc.
1265 Grey Fox Road
St. Paul, MN 55112 USA
Tel: 1 800 258 5361 (USA), +1 614 210 7300
www.smiths-medical.com
CADD, CADD-Legacy, and Smiths Medical design mark are trademarks of Smiths Medical. The symbol ® indicates the trademark is registered in the U.S. Patent and Trademark Office and certain other countries. All other names and marks mentioned are the trade names, trademarks or service marks of their respective owners.
© 2015 Smiths Medical. All rights reserved.
2015-01
40-6756-01C
- Continuous Rate: Steady delivery of DUOPA delivered throughout the day while pump is on
-
PRINCIPAL DISPLAY PANEL
NDC 0074-3012-07
Duopa®
carbidopa and levodopa
enteral suspension4.63 mg / 20 mg per mL
7 Cassettes (100 mL each)
THIS PACKAGE NOT INTENDED FOR
HOUSEHOLDS WITH YOUNG CHILDRENEach mL contains 5 mg of carbidopa monohydrate
(equivalent to 4.63 mg of carbidopa anhydrous) and 20 mg of levodopa.
Pharmacist: Store frozen.
Thaw in refrigerator prior to dispensing.See package insert for full prescribing information.
Store in the refrigerator between 36°-46°F (2°-8°C).
Protect from light.
Store cassettes in the carton until use.
Rx only
abbvie

-
INGREDIENTS AND APPEARANCE
DUOPA
carbidopa and levodopa suspensionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0074-3012 Route of Administration ENTERAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Levodopa (UNII: 46627O600J) (Levodopa - UNII:46627O600J) Levodopa 20 mg in 1 mL Carbidopa (UNII: MNX7R8C5VO) (Carbidopa Anhydrous - UNII:KR87B45RGH) Carbidopa Anhydrous 4.63 mg in 1 mL Inactive Ingredients Ingredient Name Strength CARBOXYMETHYLCELLULOSE SODIUM, UNSPECIFIED FORM (UNII: K679OBS311) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0074-3012-07 7 in 1 CARTON 01/15/2015 1 100 mL in 1 CARTRIDGE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA203952 01/15/2015 Labeler - AbbVie Inc. (078458370)