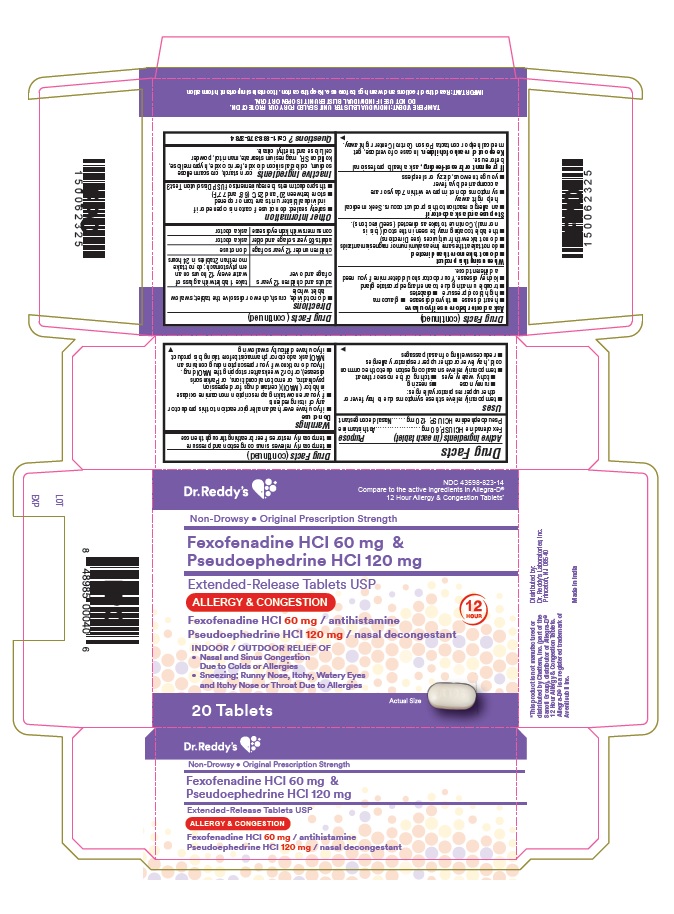
Label: FEXOFENADINE HCL AND PSEUDOEPHEDRINE HCI tablet, extended release
- NDC Code(s): 43598-823-14, 43598-823-31, 43598-823-35
- Packager: Dr. Reddy's Laboratories Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated September 9, 2019
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient(s)
- Purpose
-
Use(s)
- temporarily relieves these symptoms due to hay fever or other upper respiratory allergies:
- runny nose
- sneezing
- itchy, watery eyes
- itching of the nose or throat
- temporarily relives nasal congestion due to the common cold, hay fever or other upper respiratory allergies
- reduces swelling of nasal passages
- temporarily relieves sinus congestion and pressure
- temporarily restores freer breathing through the nose
-
Warnings
Do not use
- if you have ever had an allergic reaction to this product or any of its ingredients
- if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson’s disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
- if you have difficulty swallowing
Ask a doctor before use if you have
- heart disease
- thyroid disease
- glaucoma
- high blood pressure
- diabetes
- trouble urinating due to an enlarged prostate gland
- kidney disease. Your doctor should determine if you need a different dose.
When using this product
- do not take more than directed
- do not take at the same time as aluminum or magnesium antacids
- do not take with fruit juices (see Directions)
- the tablet coating may be seen in the stool (this is normal). Continue to take as directed (see Directions).
-
Directions
- do not divide, crush, chew or dissolve the tablet; swallow tablet whole
adults and children 12 years of age and over take 1 tablet with a glass of water every 12 hours on an empty stomach; do not take more than 2 tablets in 24 hours children under 12 years of age do not use adults 65 years of age and older ask a doctor consumers with kidney disease ask a doctor - Other information
- Inactive ingredients
- Questions?
- Principal Display Panel
-
INGREDIENTS AND APPEARANCE
FEXOFENADINE HCL AND PSEUDOEPHEDRINE HCI
fexofenadine hcl and pseudoephedrine hci tablet, extended releaseProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:43598-823 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FEXOFENADINE HYDROCHLORIDE (UNII: 2S068B75ZU) (FEXOFENADINE - UNII:E6582LOH6V) FEXOFENADINE HYDROCHLORIDE 60 mg PSEUDOEPHEDRINE HYDROCHLORIDE (UNII: 6V9V2RYJ8N) (PSEUDOEPHEDRINE - UNII:7CUC9DDI9F) PSEUDOEPHEDRINE HYDROCHLORIDE 120 mg Inactive Ingredients Ingredient Name Strength MANNITOL (UNII: 3OWL53L36A) POWDERED CELLULOSE (UNII: SMD1X3XO9M) STARCH, CORN (UNII: O8232NY3SJ) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) FERRIC OXIDE RED (UNII: 1K09F3G675) MAGNESIUM STEARATE (UNII: 70097M6I30) HYPROMELLOSES (UNII: 3NXW29V3WO) TRIETHYL CITRATE (UNII: 8Z96QXD6UM) Product Characteristics Color WHITE (buff white to pale yellow color and other layer light red to red color) Score no score Shape CAPSULE Size 16mm Flavor Imprint Code R;195 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:43598-823-14 4 in 1 CARTON 07/18/2019 1 5 in 1 BLISTER PACK; Type 0: Not a Combination Product 2 NDC:43598-823-31 6 in 1 CARTON 07/18/2019 2 5 in 1 BLISTER PACK; Type 0: Not a Combination Product 3 NDC:43598-823-35 2 in 1 CARTON 10/31/2019 3 5 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA076667 07/18/2019 Labeler - Dr. Reddy's Laboratories Inc. (802315887)