Label: TECENTRIQ- atezolizumab injection, solution
- NDC Code(s): 50242-917-01, 50242-917-86, 50242-918-01
- Packager: Genentech, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
Drug Label Information
Updated May 19, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use TECENTRIQ safely and effectively. See full prescribing information for TECENTRIQ.
TECENTRIQ® (atezolizumab) injection, for intravenous use
Initial U.S. Approval: 2016RECENT MAJOR CHANGES
INDICATIONS AND USAGE
TECENTRIQ is a programmed death-ligand 1 (PD-L1) blocking antibody indicated:
Non-Small Cell Lung Cancer (NSCLC)
- as adjuvant treatment following resection and platinum-based chemotherapy for adult patients with Stage II to IIIA NSCLC whose tumors have PD-L1 expression on ≥ 1% of tumor cells, as determined by an FDA-approved test. (1.1, 14.1)
- for the first-line treatment of adult patients with metastatic NSCLC whose tumors have high PD-L1 expression (PD-L1 stained ≥ 50% of tumor cells [TC ≥ 50%] or PD-L1 stained tumor-infiltrating immune cells [IC] covering ≥ 10% of the tumor area [IC ≥ 10%] ), as determined by an FDA-approved test, with no EGFR or ALK genomic tumor aberrations. (1.1)
- in combination with bevacizumab, paclitaxel, and carboplatin, for the first-line treatment of adult patients with metastatic non-squamous NSCLC with no EGFR or ALK genomic tumor aberrations. (1.1)
- in combination with paclitaxel protein-bound and carboplatin for the first-line treatment of adult patients with metastatic non-squamous NSCLC with no EGFR or ALK genomic tumor aberrations (1.1)
- for the treatment of adult patients with metastatic NSCLC who have disease progression during or following platinum-containing chemotherapy. Patients with EGFR or ALK genomic tumor aberrations should have disease progression on FDA-approved therapy for NSCLC harboring these aberrations prior to receiving TECENTRIQ. (1.1)
Small Cell Lung Cancer (SCLC)
- in combination with carboplatin and etoposide, for the first-line treatment of adult patients with extensive-stage small cell lung cancer (ES-SCLC). (1.2)
Hepatocellular Carcinoma (HCC)
- in combination with bevacizumab for the treatment of adult patients with unresectable or metastatic HCC who have not received prior systemic therapy. (1.3)
Melanoma
- in combination with cobimetinib and vemurafenib for the treatment of adult patients with BRAF V600 mutation-positive unresectable or metastatic melanoma. (1.4)
Alveolar Soft Part Sarcoma (ASPS)
- for the treatment of adult and pediatric patients 2 years of age and older with unresectable or metastatic ASPS. (1.5)
DOSAGE AND ADMINISTRATION
Administer TECENTRIQ intravenously over 60 minutes. If the first infusion is tolerated, all subsequent infusions may be delivered over 30 minutes.
NSCLC
- In the adjuvant setting, administer TECENTRIQ following resection and up to 4 cycles of platinum-based chemotherapy as 840 mg every 2 weeks, 1200 mg every 3 weeks or 1680 mg every 4 weeks for up to 1 year. (2.2)
- In the metastatic setting, administer TECENTRIQ as 840 mg every 2 weeks, 1200 mg every 3 weeks, or 1680 mg every 4 weeks. (2.2)
- When administering with chemotherapy with or without bevacizumab, administer TECENTRIQ prior to chemotherapy and bevacizumab when given on the same day. (2.2)
Small Cell Lung Cancer
- Administer TECENTRIQ as 840 mg every 2 weeks, 1200 mg every 3 weeks, or 1680 mg every 4 weeks. When administering with carboplatin and etoposide, administer TECENTRIQ prior to chemotherapy when given on the same day. (2.2)
Hepatocellular Carcinoma
- Administer TECENTRIQ as 840 mg every 2 weeks, 1200 mg every 3 weeks, or 1680 mg every 4 weeks. Administer TECENTRIQ prior to bevacizumab when given on the same day. Bevacizumab is administered at 15 mg/kg every 3 weeks. (2.2)
Melanoma
- Following completion of a 28 day cycle of cobimetinib and vemurafenib, administer TECENTRIQ 840 mg every 2 weeks, 1200 mg every 3 weeks, or 1680 mg every 4 weeks with cobimetinib 60 mg orally once daily (21 days on /7 days off) and vemurafenib 720 mg orally twice daily. (2.2)
ASPS
DOSAGE FORMS AND STRENGTHS
Injection: 840 mg/14 mL (60 mg/mL) and 1200 mg/20 mL (60 mg/mL) solution in a single-dose vial. (3)
CONTRAINDICATIONS
None. (4)
WARNINGS AND PRECAUTIONS
-
Immune-Mediated Adverse Reactions
- Immune-mediated adverse reactions, which may be severe or fatal, can occur in any organ system or tissue, including the following: immune-mediated pneumonitis, immune-mediated colitis, immune-mediated hepatitis, immune-mediated endocrinopathies, immune-mediated dermatologic adverse reactions, immune-mediated nephritis and renal dysfunction, and solid organ transplant rejection. (5.1)
- Monitor for early identification and management. Evaluate liver enzymes, creatinine, and thyroid function at baseline and periodically during treatment. (5.1)
- Withhold or permanently discontinue based on severity and type of reaction. (5.1).
- Infusion-Related Reactions: Interrupt, slow the rate of infusion, or permanently discontinue based on severity of infusion reactions. (5.2)
- Complications of Allogeneic HSCT: Fatal and other serious complications can occur in patients who receive allogeneic HSCT before or after being treated with a PD-1/PD-L1 blocking antibody. (5.3)
- Embryo-Fetal Toxicity: Can cause fetal harm. Advise females of reproductive potential of the potential risk to a fetus and use of effective contraception. (5.4, 8.1, 8.3)
ADVERSE REACTIONS
TECENTRIQ as a single-agent
- Most common adverse reactions (≥ 20%) with TECENTRIQ as a single-agent are fatigue/asthenia, decreased appetite, nausea, cough, and dyspnea. (6.1)
TECENTRIQ in combination with other antineoplastic drugs
- Most common adverse reactions (≥ 20%) in patients with NSCLC and SCLC are fatigue/asthenia, nausea, alopecia, constipation, diarrhea, and decreased appetite. (6.1)
TECENTRIQ in combination with bevacizumab
- Most common adverse reactions (≥ 20%) in patients with HCC are hypertension, fatigue and proteinuria. (6.1)
TECENTRIQ in combination with cobimetinib and vemurafenib
- Most common adverse reactions (≥ 20%) with TECENTRIQ in patients with melanoma are rash, musculoskeletal pain, fatigue, hepatotoxicity, pyrexia, nausea, pruritus, edema, stomatitis, hypothyroidism, and photosensitivity reaction. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Genentech at 1-888-835-2555 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 4/2023
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
1.1 Non-Small Cell Lung Cancer
1.2 Small Cell Lung Cancer
1.3 Hepatocellular Carcinoma
1.4 Melanoma
1.5 Alveolar Soft Part Sarcoma
2 DOSAGE AND ADMINISTRATION
2.1 Patient Selection for Treatment of Non-Small Cell Lung Cancer and Melanoma
2.2 Recommended Dosage
2.3 Dosage Modifications for Adverse Reactions
2.4 Preparation and Administration
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Severe and Fatal Immune-Mediated Adverse Reactions
5.2 Infusion-Related Reactions
5.3 Complications of Allogeneic HSCT after PD-1/PD-L1 Inhibitors
5.4 Embryo-Fetal Toxicity
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
12.6 Immunogenicity
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
13.2 Animal Toxicology and/or Pharmacology
14 CLINICAL STUDIES
14.1 Non-Small Cell Lung Cancer
14.2 Small Cell Lung Cancer
14.3 Hepatocellular Carcinoma
14.4 Melanoma
14.5 Alveolar soft part sarcoma (ASPS)
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
1.1 Non-Small Cell Lung Cancer
- TECENTRIQ, as a single-agent, is indicated as adjuvant treatment following resection and platinum-based chemotherapy for adult patients with stage II to IIIA [see Clinical Studies (14.1)] non-small cell lung cancer (NSCLC) whose tumors have PD-L1 expression on ≥ 1% of tumor cells, as determined by an FDA-approved test [see Dosage and Administration (2.1)].
- TECENTRIQ, as a single agent, is indicated for the first-line treatment of adult patients with metastatic non-small cell lung cancer (NSCLC) whose tumors have high PD-L1 expression (PD-L1 stained ≥ 50% of tumor cells [TC ≥ 50%] or PD-L1 stained tumor-infiltrating immune cells [IC] covering ≥ 10% of the tumor area [IC ≥ 10%]), as determined by an FDA-approved test, with no EGFR or ALK genomic tumor aberrations [see Dosage and Administration (2.1)].
- TECENTRIQ, in combination with bevacizumab, paclitaxel, and carboplatin, is indicated for the first-line treatment of adult patients with metastatic non-squamous NSCLC with no EGFR or ALK genomic tumor aberrations.
- TECENTRIQ, in combination with paclitaxel protein-bound and carboplatin, is indicated for the first-line treatment of adult patients with metastatic non-squamous NSCLC with no EGFR or ALK genomic tumor aberrations.
- TECENTRIQ, as a single-agent, is indicated for the treatment of adult patients with metastatic NSCLC who have disease progression during or following platinum-containing chemotherapy. Patients with EGFR or ALK genomic tumor aberrations should have disease progression on FDA-approved therapy for NSCLC harboring these aberrations prior to receiving TECENTRIQ.
1.2 Small Cell Lung Cancer
TECENTRIQ, in combination with carboplatin and etoposide, is indicated for the first-line treatment of adult patients with extensive-stage small cell lung cancer (ES-SCLC).
1.3 Hepatocellular Carcinoma
TECENTRIQ, in combination with bevacizumab, is indicated for the treatment of adult patients with unresectable or metastatic hepatocellular carcinoma (HCC) who have not received prior systemic therapy.
1.4 Melanoma
TECENTRIQ, in combination with cobimetinib and vemurafenib, is indicated for the treatment of adult patients with BRAF V600 mutation-positive unresectable or metastatic melanoma [see Dosage and Administration (2.1)].
-
2 DOSAGE AND ADMINISTRATION
2.1 Patient Selection for Treatment of Non-Small Cell Lung Cancer and Melanoma
Select patients with Stage II to IIIA non-small cell lung cancer for treatment with TECENTRIQ as a single agent based on PD-L1 expression on tumor cells [see Clinical Studies (14.1)].
Select patients with first-line metastatic non-small cell lung cancer for treatment with TECENTRIQ as a single agent based on the PD-L1 expression on tumor cells or on tumor-infiltrating immune cells [see Clinical Studies (14.1)].
Information on FDA-approved tests for the determination of PD-L1 expression in metastatic non-small cell lung cancer are available at: http://www.fda.gov/CompanionDiagnostics.
Select patients with unresectable or metastatic melanoma for treatment with TECENTRIQ in combination with cobimetinib and vemurafenib after confirming the presence of a BRAF V600 mutation [see Clinical Studies (14.4)]. Information on FDA-approved tests for the detection of BRAF V600 mutations in melanoma is available at: http://www.fda.gov/CompanionDiagnostics.
2.2 Recommended Dosage
The recommended dosages of TECENTRIQ administered intravenously as a single agent are presented in Table 1.
Table 1: Recommended Dosage of TECENTRIQ as a Single Agent * 60-minute intravenous infusion. If the first infusion is tolerated, all subsequent infusions may be delivered over 30 minutes. Metastatic NSCLC - 840 mg every 2 weeks or
- 1200 mg every 3 weeks or
- 1680 mg every 4 weeks
Until disease progression or unacceptable toxicity Adjuvant Treatment of NSCLC - 840 mg every 2 weeks or
- 1200 mg every 3 weeks or
- 1680 mg every 4 weeks
Up to one year, unless there is disease recurrence or unacceptable toxicity ASPS (adult) - 840 mg every 2 weeks or
- 1200 mg every 3 weeks or
- 1680 mg every 4 weeks
Until disease progression or unacceptable toxicity ASPS (pediatric, 2 years of age and older) 15 mg/kg (up to a maximum 1200 mg) every 3 weeks The recommended intravenous dosages of TECENTRIQ in combination with other therapeutic agents are presented in Table 2. Refer to the respective Prescribing Information for each therapeutic agent administered in combination with TECENTRIQ for the recommended dosage information, as appropriate.
Table 2: Recommended Dosage of TECENTRIQ in Combination with Other Therapeutic Agents Indication Recommended Dosage of TECENTRIQ* Duration of Therapy - *
- 60-minute intravenous infusion. If the first infusion is tolerated, all subsequent infusions may be delivered over 30 minutes.
NSCLC - 840 mg every 2 weeks or
- 1200 mg every 3 weeks or
- 1680 mg every 4 weeks
Until disease progression or unacceptable toxicity SCLC - 840 mg every 2 weeks or
- 1200 mg every 3 weeks or
- 1680 mg every 4 weeks
HCC - 840 mg every 2 weeks or
- 1200 mg every 3 weeks or
- 1680 mg every 4 weeks
Melanoma - 840 mg every 2 weeks or
- 1200 mg every 3 weeks or
- 1680 mg every 4 weeks
Prior to initiating TECENTRIQ, patients should receive a 28 day treatment cycle of cobimetinib 60 mg orally once daily (21 days on and 7 days off) and vemurafenib 960 mg orally twice daily from Days 1-21 and vemurafenib 720 mg orally twice daily from Days 22-28.2.3 Dosage Modifications for Adverse Reactions
No dose reduction for TECENTRIQ is recommended. In general, withhold TECENTRIQ for severe (Grade 3) immune-mediated adverse reactions. Permanently discontinue TECENTRIQ for life-threatening (Grade 4) immune-mediated adverse reactions, recurrent severe (Grade 3) immune-mediated reactions that require systemic immunosuppressive treatment, or an inability to reduce corticosteroid dose to 10 mg or less of prednisone or equivalent per day within 12 weeks of initiating steroids.
Dosage modifications for TECENTRIQ for adverse reactions that require management different from these general guidelines are summarized in Table 3.
Table 3: Recommended Dosage Modifications for Adverse Reactions Adverse Reaction Severity* Dosage Modification ALT = alanine aminotransferase, AST = aspartate aminotransferase, ULN = upper limit normal, DRESS = Drug Rash with Eosinophilia and Systemic Symptoms, SJS = Stevens Johnson syndrome, TEN = toxic epidermal necrolysis - *
- Based on Common Terminology Criteria for Adverse Events (CTCAE), version 4
- †
- Resume in patients with complete or partial resolution (Grade 0 to 1) after corticosteroid taper. Permanently discontinue if no complete or partial resolution within 12 weeks of initiating steroids or inability to reduce prednisone to 10 mg per day or less (or equivalent) within 12 weeks of initiating steroids
- ‡
- If AST and ALT are less than or equal to ULN at baseline, withhold or permanently discontinue TECENTRIQ based on recommendations for hepatitis with no liver involvement
Immune-Mediated Adverse Reactions [see Warnings and Precautions (5.1)] Pneumonitis Grade 2 Withhold† Grades 3 or 4 Permanently discontinue Colitis Grades 2 or 3 Withhold† Grade 4 Permanently discontinue Hepatitis with no tumor involvement of the liver AST or ALT increases to more than 3 and up to 8 times ULN
or
Total bilirubin increases to more than 1.5 and up to 3 times ULNWithhold† AST or ALT increases to more than 8 times ULN
or
Total bilirubin increases to more than 3 times ULNPermanently discontinue Hepatitis with tumor involvement of the liver‡ Baseline AST or ALT is more than 1 and up to 3 times ULN and increases to more than 5 and up to 10 times ULN
or
Baseline AST or ALT is more than 3 and up to 5 times ULN and increases to more than 8 and up to 10 times ULNWithhold† AST or ALT increases to more than 10 times ULN
or
Total bilirubin increases to more than 3 times ULNPermanently discontinue Endocrinopathies Grades 3 or 4 Withhold until clinically stable or permanently discontinue depending on severity Nephritis with Renal Dysfunction Grades 2 or 3 increased blood creatinine Withhold† Grade 4 increased blood creatinine Permanently discontinue Exfoliative Dermatologic Conditions Suspected SJS, TEN, or DRESS Withhold Confirmed SJS, TEN, or DRESS Permanently discontinue Myocarditis or Pericarditis Grades 2, 3, or 4 Permanently discontinue Neurological Toxicities Grade 2 Withhold† Grades 3 or 4 Permanently discontinue Other Adverse Reactions Infusion-Related Reactions [see Warnings and Precautions (5.2)] Grades 1 or 2 Interrupt or slow the rate of infusion Grades 3 or 4 Permanently discontinue 2.4 Preparation and Administration
Preparation
Visually inspect drug product for particulate matter and discoloration prior to administration, whenever solution and container permit. Discard the vial if the solution is cloudy, discolored, or visible particles are observed. Do not shake the vial.
Prepare the solution for infusion as follows:
- Select the appropriate vial(s) based on the prescribed dose.
- Withdraw the required volume of TECENTRIQ from the vial(s) using sterile needle and syringe.
- Dilute to a final concentration between 3.2 mg/mL and 16.8 mg/mL in a polyvinyl chloride (PVC), polyethylene (PE), or polyolefin (PO) infusion bag containing 0.9% Sodium Chloride Injection, USP.
- Dilute with only 0.9% Sodium Chloride Injection, USP.
- Mix diluted solution by gentle inversion. Do not shake.
- Discard used or empty vials of TECENTRIQ.
Storage of Infusion Solution
This product does not contain a preservative.
Administer immediately once prepared. If diluted TECENTRIQ infusion solution is not used immediately, store solution either:
- At room temperature for no more than 6 hours from the time of preparation. This includes room temperature storage of the infusion in the infusion bag and time for administration of the infusion, or
- Under refrigeration at 2°C to 8°C (36°F to 46°F) for no more than 24 hours from time of preparation.
Do not freeze.
Do not shake.
Administration
Administer the initial infusion over 60 minutes through an intravenous line with or without a sterile, non-pyrogenic, low-protein binding in-line filter (pore size of 0.2–0.22 micron). If the first infusion is tolerated, all subsequent infusions may be delivered over 30 minutes.
Do not coadminister other drugs through the same intravenous line.
Do not administer as an intravenous push or bolus.
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Severe and Fatal Immune-Mediated Adverse Reactions
TECENTRIQ is a monoclonal antibody that belongs to a class of drugs that bind to either the programmed death-receptor 1 (PD-1) or the PD-ligand 1 (PD-L1), blocking the PD-1/PD-L1 pathway, thereby removing inhibition of the immune response, potentially breaking peripheral tolerance and inducing immune-mediated adverse reactions. Important immune-mediated adverse reactions listed under Warnings and Precautions may not include all possible severe and fatal immune-mediated reactions.
Immune-mediated adverse reactions, which may be severe or fatal, can occur in any organ system or tissue. Immune-mediated adverse reactions can occur at any time after starting a PD-1/PD-L1 blocking antibody. While immune-mediated adverse reactions usually manifest during treatment with PD-1/PD-L1 blocking antibodies, immune-mediated adverse reactions can also manifest after discontinuation of PD-1/PD-L1 blocking antibodies.
Early identification and management of immune-mediated adverse reactions are essential to ensure safe use of PD-1/PD-L1 blocking antibodies. Monitor patients closely for symptoms and signs that may be clinical manifestations of underlying immune-mediated adverse reactions. Evaluate liver enzymes, creatinine, and thyroid function at baseline and periodically during treatment. In cases of suspected immune-mediated adverse reactions, initiate appropriate workup to exclude alternative etiologies, including infection. Institute medical management promptly, including specialty consultation as appropriate.
Withhold or permanently discontinue TECENTRIQ depending on severity [see Dosage and Administration (2.3)]. In general, if TECENTRIQ requires interruption or discontinuation, administer systemic corticosteroid therapy (1 to 2 mg/kg/day prednisone or equivalent) until improvement to Grade 1 or less. Upon improvement to Grade 1 or less, initiate corticosteroid taper and continue to taper over at least 1 month. Consider administration of other systemic immunosuppressants in patients whose immune-mediated adverse reactions are not controlled with corticosteroid therapy.
Toxicity management guidelines for adverse reactions that do not necessarily require systemic steroids (e.g., endocrinopathies and dermatologic reactions) are discussed below.
Immune-Mediated Pneumonitis
TECENTRIQ can cause immune-mediated pneumonitis. The incidence of pneumonitis is higher in patients who have received prior thoracic radiation.
TECENTRIQ as a Single Agent:
Immune-mediated pneumonitis occurred in 3% (83/2616) of patients receiving TECENTRIQ as a single agent, including fatal (<0.1%), Grade 4 (0.2%), Grade 3 (0.8%), and Grade 2 (1.1%) adverse reactions. Pneumonitis led to permanent discontinuation of TECENTRIQ in 0.5% and withholding of TECENTRIQ in 1.5% of patients.
Systemic corticosteroids were required in 55% (46/83) of patients with pneumonitis. Pneumonitis resolved in 69% of the 83 patients. Of the 39 patients in whom TECENTRIQ was withheld for pneumonitis, 25 reinitiated TECENTRIQ after symptom improvement; of these, 4% had recurrence of pneumonitis.
In IMpower010 immune-mediated pneumonitis occurred in 3.8% (19/495) of patients receiving TECENTRIQ as a single agent, including fatal (0.2%), Grade 4 (0.2%), and Grade 3 (0.6%) adverse reactions. Pneumonitis led to permanent discontinuation of TECENTRIQ in 2.2% and withholding of TECENTRIQ in 0.8% of patients.
Systemic corticosteroids were required in 63% (12/19) of patients with pneumonitis. Pneumonitis resolved in 84% of the 19 patients.
TECENTRIQ in Combination with Cobimetinib and Vemurafenib:
Immune-mediated pneumonitis occurred in 13% (29/230) of patients receiving TECENTRIQ in combination with cobimetinib and vemurafenib, including Grade 3 (1.3%) and Grade 2 (7%) adverse reactions. Pneumonitis led to permanent discontinuation of TECENTRIQ in 2.6% and withholding of TECENTRIQ in 7.4% of patients.
Systemic corticosteroids were required in 55% (16/29) of patients with pneumonitis. Pneumonitis resolved in 97% of the 29 patients. Of the 17 patients in whom TECENTRIQ was withheld for pneumonitis, 10 reinitiated TECENTRIQ after symptom improvement; of these, 50% had recurrence of pneumonitis.
Immune-Mediated Colitis
TECENTRIQ can cause immune-mediated colitis. Colitis can present with diarrhea, abdominal pain, and lower gastrointestinal (GI) bleeding. Cytomegalovirus (CMV) infection/reactivation has been reported in patients with corticosteroid-refractory immune-mediated colitis. In cases of corticosteroid-refractory colitis, consider repeating infectious workup to exclude alternative etiologies.
TECENTRIQ as a Single Agent:
Immune-mediated colitis occurred in 1% (26/2616) of patients receiving TECENTRIQ as a single agent, including Grade 3 (0.5%) and Grade 2 (0.3%) adverse reactions. Colitis led to permanent discontinuation of TECENTRIQ in 0.2% and withholding of TECENTRIQ in 0.5% of patients.
Systemic corticosteroids were required in 50% (13/26) of patients with colitis. Colitis resolved in 73% of the 26 patients. Of the 12 patients in whom TECENTRIQ was withheld for colitis, 8 reinitiated treatment with TECENTRIQ after symptom improvement; of these, 25% had recurrence of colitis.
Immune-Mediated Hepatitis
TECENTRIQ can cause immune-mediated hepatitis.
Immune-mediated hepatitis occurred in 1.8% (48/2616) of patients receiving TECENTRIQ as a single agent, including fatal (<0.1%), Grade 4 (0.2%), Grade 3 (0.5%), and Grade 2 (0.5%) adverse reactions. Hepatitis led to permanent discontinuation of TECENTRIQ in 0.2% and withholding of TECENTRIQ in 0.2% of patients.
Systemic corticosteroids were required in 25% (12/48) of patients with hepatitis. Hepatitis resolved in 50% of the 48 patients. Of the 6 patients in whom TECENTRIQ was withheld for hepatitis, 4 reinitiated treatment with TECENTRIQ after symptom improvement; of these, none had recurrence of hepatitis.
TECENTRIQ in Combination with Cobimetinib and Vemurafenib:
Immune-mediated hepatitis occurred in 6.1% (14/230) of patients receiving TECENTRIQ in combination with cobimetinib and vemurafenib, including Grade 4 (1.3%), Grade 3 (1.7%) and Grade 2 (1.3%) adverse reactions. Hepatitis led to permanent discontinuation of TECENTRIQ in 2.2% and withholding of TECENTRIQ in 1.7% of patients.
Systemic corticosteroids were required in 50% (7/14) of patients with hepatitis. Hepatitis resolved in 93% of the 14 patients. Of the 4 patients in whom TECENTRIQ was withheld for hepatitis, 3 reinitiated TECENTRIQ after symptom improvement; of these, 33% had recurrence of hepatitis.
Immune-Mediated Endocrinopathies
Adrenal Insufficiency
TECENTRIQ can cause primary or secondary adrenal insufficiency. For Grade 2 or higher adrenal insufficiency, initiate symptomatic treatment, including hormone replacement as clinically indicated. Withhold or permanently discontinue TECENTRIQ depending on severity [see Dosage and Administration (2.3)].
Adrenal insufficiency occurred in 0.4% (11/2616) of patients receiving TECENTRIQ as a single agent, including Grade 3 (<0.1%) and Grade 2 (0.2%) adverse reactions. Adrenal insufficiency led to permanent discontinuation of TECENTRIQ in one patient and withholding of TECENTRIQ in one patient.
Systemic corticosteroids were required in 82% (9/11) of patients with adrenal insufficiency; of these, 3 patients remained on systemic corticosteroids. The single patient in whom TECENTRIQ was withheld for adrenal insufficiency did not reinitiate TECENTRIQ.
In IMpower010 immune-mediated adrenal insufficiency occurred in 1.2% (6/495) of patients receiving TECENTRIQ as a single agent, including Grade 3 (0.4%) adverse reactions. Adrenal insufficiency led to permanent discontinuation of TECENTRIQ in 0.6% and withholding of TECENTRIQ in 0.2% of patients.
Systemic corticosteroids were required in 83% (5/6) of patients with adrenal insufficiency; of these, 4 patients remained on systemic corticosteroids.
Hypophysitis
TECENTRIQ can cause immune-mediated hypophysitis. Hypophysitis can present with acute symptoms associated with mass effect such as headache, photophobia, or visual field cuts. Hypophysitis can cause hypopituitarism. Initiate hormone replacement as clinically indicated. Withhold or permanently discontinue TECENTRIQ depending on severity [see Dosage and Administration (2.3)].
Hypophysitis occurred in <0.1% (2/2616) of patients receiving TECENTRIQ as a single agent, including Grade 2 (1 patient, <0.1%) adverse reactions. Hypophysitis led to permanent discontinuation of TECENTRIQ in one patient and no patients required withholding of TECENTRIQ.
Systemic corticosteroids were required in 50% (1/2) of patients with hypophysitis. Hypophysitis did not resolve in these 2 patients.
Thyroid disorders
TECENTRIQ can cause immune-mediated thyroid disorders. Thyroiditis can present with or without endocrinopathy. Hypothyroidism can follow hyperthyroidism. Initiate hormone replacement for hypothyroidism or medical management for hyperthyroidism as clinically indicated. Withhold or permanently discontinue TECENTRIQ depending on severity [see Dosage and Administration (2.3)].
Thyroiditis:
Thyroiditis occurred in 0.2% (4/2616) of patients receiving TECENTRIQ as a single agent, including Grade 2 (<0.1%) adverse reactions. Thyroiditis did not lead to permanent discontinuation of TECENTRIQ in any of these patients, but led to withholding of TECENTRIQ in one patient.
Hormone replacement therapy was required in 75% (3/4) of patients with thyroiditis. Systemic corticosteroids were required in 25% (1/4) of patients with thyroiditis. Thyroiditis resolved in 50% of patients. The single patient in whom TECENTRIQ was withheld for thyroiditis reinitiated TECENTRIQ; this patient did not have recurrence of thyroiditis.
In IMpower010, thyroiditis occurred in 1.2% (6/495) of patients receiving TECENTRIQ as a single agent, including Grade 2 (0.4%) adverse reactions. Thyroiditis led to withholding of TECENTRIQ in one patient.
Hormone replacement therapy was required in 67% (4/6) of patients with thyroiditis. Systemic corticosteroids were required in 33% (2/6) of patients with thyroiditis. Thyroiditis resolved in 50% of patients.
Hyperthyroidism:
TECENTRIQ as a Single Agent:
Hyperthyroidism occurred in 0.8% (21/2616) of patients receiving TECENTRIQ as a single agent, including Grade 2 (0.4%) adverse reactions. Hyperthyroidism did not lead to permanent discontinuation of TECENTRIQ in any of these patients, but led to withholding of TECENTRIQ in 0.1% of patients.
Antithyroid therapy was required in 29% (6/21) of patients with hyperthyroidism. Of these 6 patients, the majority remained on antithyroid treatment. Of the 3 patients in whom TECENTRIQ was withheld for hyperthyroidism, one patient reinitiated TECENTRIQ; this patient did not have recurrence of hyperthyroidism.
In IMpower010 hyperthyroidism occurred in 6% (32/495) of patients receiving TECENTRIQ as a single agent, including Grade 3 (0.4%) adverse reactions. Hyperthyroidism led to permanent discontinuation of TECENTRIQ in 0.8% and withholding of TECENTRIQ in 2.8% of patients.
Antithyroid therapy was required in 38% (12/32) of patients with hyperthyroidism. Of these 12 patients, the majority remained on antithyroid treatment. Of the 14 patients in whom TECENTRIQ was withheld for hyperthyroidism, 9 patients reinitiated TECENTRIQ.
TECENTRIQ in Combination with Cobimetinib and Vemurafenib:
Hyperthyroidism occurred in 19% (43/230) of patients receiving TECENTRIQ in combination with cobimetinib and vemurafenib, including Grade 3 (0.9%) and Grade 2 (7.8%) adverse reactions. Hyperthyroidism led to permanent discontinuation of TECENTRIQ in 0.4% and withholding of TECENTRIQ in 10% of patients.
Antithyroid therapy was required in 53% (23/43) of patients with hyperthyroidism. Of these 23 patients, the majority remained on antithyroid treatment. Of the 24 patients in whom TECENTRIQ was withheld for hyperthyroidism, 18 patients reinitiated TECENTRIQ; of these, 28% had recurrence of hyperthyroidism.
Hypothyroidism:
TECENTRIQ as a Single Agent:
Hypothyroidism occurred in 4.9% (128/2616) of patients receiving TECENTRIQ as a single agent, including Grade 3 (0.2%) and Grade 2 (3.4%) adverse reactions. Hypothyroidism did not lead to permanent discontinuation of TECENTRIQ in any of these patients, but led to withholding of TECENTRIQ in 0.6% of patients.
Hormone replacement therapy was required in 81% (104/128) of patients with hypothyroidism. The majority of patients with hypothyroidism remained on thyroid hormone replacement. Of the 17 patients in whom TECENTRIQ was withheld for hypothyroidism, 8 reinitiated TECENTRIQ after symptom improvement.
In IMpower010 hypothyroidism occurred in 17% (86/495) of patients receiving TECENTRIQ as a single agent. Hypothyroidism led to permanent discontinuation of TECENTRIQ in 1.6% and withholding of TECENTRIQ in 1.6% of patients.
Hormone replacement was required in 57% (49/86) of patients with hypothyroidism. The majority of patients with hypothyroidism remained on thyroid hormone replacement. Of the 8 patients in whom TECENTRIQ was withheld for hypothyroidism, 3 reinitiated TECENTRIQ after symptom improvement.
TECENTRIQ in Combination with Platinum-based Chemotherapy:
Hypothyroidism occurred in 11% (277/2421) of patients with NSCLC and SCLC receiving TECENTRIQ in combination with platinum-based chemotherapy, including Grade 4 (<0.1%), Grade 3 (0.3%), and Grade 2 (5.7%) adverse reactions. Hypothyroidism led to permanent discontinuation of TECENTRIQ in 0.1% and withholding of TECENTRIQ in 1.6% of patients.
Hormone replacement therapy was required in 71% (198/277) of patients with hypothyroidism. The majority of patients with hypothyroidism remained on thyroid hormone replacement. Of the 39 patients in whom TECENTRIQ was withheld for hypothyroidism, 9 reinitiated TECENTRIQ after symptom improvement.
TECENTRIQ in Combination with Cobimetinib and Vemurafenib:
Hypothyroidism occurred in 26% (60/230) of patients receiving TECENTRIQ in combination with cobimetinib and vemurafenib, including Grade 2 (9.1%) adverse reactions. Hypothyroidism did not lead to permanent discontinuation of TECENTRIQ in any of these patients, but led to withholding of TECENTRIQ in 2.6% of patients.
Hormone replacement therapy was required in 52% (31/60) of patients with hypothyroidism. The majority of patients with hypothyroidism remained on thyroid hormone replacement. Of the 6 patients in whom TECENTRIQ was withheld for hypothyroidism, 4 reinitiated TECENTRIQ after symptom improvement. The majority of patients with hypothyroidism required long term thyroid replacement.
Type 1 Diabetes Mellitus, which can present with Diabetic Ketoacidosis
Monitor patients for hyperglycemia or other signs and symptoms of diabetes. Initiate treatment with insulin as clinically indicated. Withhold or permanently discontinue TECENTRIQ depending on severity [see Dosage and Administration (2.3)].
Type 1 diabetes mellitus occurred in 0.3% (7/2616) of patients receiving TECENTRIQ, including Grade 3 (0.2%) and Grade 2 (<0.1%) adverse reactions. Type 1 diabetes mellitus led to permanent discontinuation of TECENTRIQ in one patient and withholding of TECENTRIQ in two patients.
Treatment with insulin was required for all patients with confirmed Type 1 diabetes mellitus and insulin therapy was continued long-term. Of the 2 patients in whom TECENTRIQ was withheld for Type 1 diabetes mellitus, both re-initiated TECENTRIQ treatment.
Immune-Mediated Nephritis with Renal Dysfunction
TECENTRIQ can cause immune-mediated nephritis.
TECENTRIQ as a Single Agent:
Immune-mediated nephritis with renal dysfunction occurred in <0.1% (1/2616) of patients receiving TECENTRIQ as a single agent, and this adverse reaction was a Grade 3 (<0.1%) adverse reaction. Nephritis led to permanent discontinuation of TECENTRIQ in this patient.
This patient required systemic corticosteroids. In this patient, nephritis did not resolve.
TECENTRIQ in Combination with Cobimetinib and Vemurafenib:
Immune-mediated nephritis with renal dysfunction occurred in 1.3% (3/230) of patients receiving TECENTRIQ in combination with cobimetinib and vemurafenib, including Grade 2 (1.3%) adverse reactions. Nephritis led to permanent discontinuation of TECENTRIQ in 0.4% and withholding of TECENTRIQ in 0.9% of patients.
Systemic corticosteroids were required in 67% (2/3) of patients with nephritis. Nephritis resolved in all 3 of these patients. Of the 2 patients in whom TECENTRIQ was withheld for nephritis, both reinitiated TECENTRIQ after symptom improvement and neither had recurrence of nephritis.
Immune-Mediated Dermatologic Adverse Reactions
TECENTRIQ can cause immune-mediated rash or dermatitis. Exfoliative dermatitis, including Stevens-Johnson syndrome (SJS), DRESS, and toxic epidermal necrolysis (TEN), has occurred with PD-1/PD-L1 blocking antibodies. Topical emollients and/or topical corticosteroids may be adequate to treat mild to moderate non-exfoliative rashes. Withhold or permanently discontinue TECENTRIQ depending on severity [see Dosage and Administration (2.3)].
Immune-mediated dermatologic adverse reactions occurred in 0.6% (15/2616) of patients receiving TECENTRIQ as a single agent, including Grade 3 (<0.1%) and Grade 2 (0.2%) adverse reactions. Dermatologic adverse reactions led to permanent discontinuation of TECENTRIQ in 0.1% and withholding of TECENTRIQ in 0.2% of patients.
Systemic corticosteroids were required in 20% (3/15) of patients with dermatologic adverse reactions. Dermatologic adverse reactions resolved in 87% of the 15 patients. Of the 4 patients in whom TECENTRIQ was withheld for immune-mediated dermatologic adverse reactions, none re-initiated TECENTRIQ.
Other Immune-Mediated Adverse Reactions
The following clinically significant immune-mediated adverse reactions occurred at an incidence of < 1% (unless otherwise noted) in patients who received TECENTRIQ or were reported with the use of other PD-1/PD-L1 blocking antibodies. Severe or fatal cases have been reported for some of these adverse reactions.
Cardiac/Vascular: Myocarditis, pericarditis, vasculitis.
Nervous System: Meningitis, encephalitis, myelitis and demyelination, myasthenic syndrome/myasthenia gravis (including exacerbation), Guillain-Barré syndrome, nerve paresis, autoimmune neuropathy.
Ocular: Uveitis, iritis, and other ocular inflammatory toxicities can occur. Some cases can be associated with retinal detachment. Various grades of visual impairment, including blindness, can occur. If uveitis occurs in combination with other immune-mediated adverse reactions, consider a Vogt-Koyanagi-Harada-like syndrome, as this may require treatment with systemic steroids to reduce the risk of permanent vision loss.
Gastrointestinal: Pancreatitis to include increases in serum amylase and lipase levels, gastritis, duodenitis.
Musculoskeletal and Connective Tissue: Myositis/polymyositis, rhabdomyolysis and associated sequelae including renal failure, arthritis, polymyalgia rheumatic.
Endocrine: Hypoparathyroidism.
Other (Hematologic/Immune): Hemolytic anemia, aplastic anemia, hemophagocytic lymphohistiocytosis, systemic inflammatory response syndrome, histiocytic necrotizing lymphadenitis (Kikuchi lymphadenitis), sarcoidosis, immune thrombocytopenic purpura, solid organ transplant rejection.
5.2 Infusion-Related Reactions
TECENTRIQ can cause severe or life-threatening infusion-related reactions. Monitor for signs and symptoms of infusion-related reactions. Interrupt, slow the rate of, or permanently discontinue TECENTRIQ based on the severity [see Dosage and Administration (2.3)]. For Grade 1 or 2 infusion-related reactions, consider using pre-medications with subsequent doses.
In clinical studies enrolling 2616 patients with various cancers who received TECENTRIQ as a single-agent [see Adverse Reactions (6.1)], infusion-related reactions occurred in 1.3% of patients, including Grade 3 (0.2%). The frequency and severity of infusion-related reactions were similar whether TECENTRIQ was given as a single-agent in patients with various cancers, in combination with other antineoplastic drugs in NSCLC and SCLC, and across the recommended dose range (840 mg Q2W to 1680 mg Q4W).
5.3 Complications of Allogeneic HSCT after PD-1/PD-L1 Inhibitors
Fatal and other serious complications can occur in patients who receive allogeneic hematopoietic stem cell transplantation (HSCT) before or after being treated with a PD-1/PD-L1 blocking antibody. Transplant-related complications include hyperacute graft-versus-host disease (GVHD), acute GVHD, chronic GVHD, hepatic veno-occlusive disease (VOD) after reduced intensity conditioning, and steroid-requiring febrile syndrome (without an identified infectious cause). These complications may occur despite intervening therapy between PD-1/PD-L1 blockage and allogeneic HSCT.
Follow patients closely for evidence of transplant-related complications and intervene promptly. Consider the benefits versus risks of treatment with a PD-1/PD-L1 blocking antibody prior to or after an allogeneic HSCT.
5.4 Embryo-Fetal Toxicity
Based on its mechanism of action, TECENTRIQ can cause fetal harm when administered to a pregnant woman. There are no available data on the use of TECENTRIQ in pregnant women. Animal studies have demonstrated that inhibition of the PD-L1/PD-1 pathway can lead to increased risk of immune-related rejection of the developing fetus resulting in fetal death.
Verify pregnancy status of females of reproductive potential prior to initiating TECENTRIQ. Advise females of reproductive potential of the potential risk to a fetus. Advise females of reproductive potential to use effective contraception during treatment with TECENTRIQ and for at least 5 months after the last dose [see Use in Specific Populations (8.1, 8.3)].
-
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in the labeling:
- Severe and Fatal Immune-Mediated Adverse Reactions [see Warnings and Precautions (5.1)]
- Infusion-Related Reactions [see Warnings and Precautions (5.2)]
- Complications of Allogeneic HSCT after PD-1/PD-L1 Inhibitors [see Warnings and Precautions (5.3)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The data described in WARNINGS AND PRECAUTIONS reflect exposure to TECENTRIQ as a single-agent in 2616 patients in two randomized, active-controlled studies (POPLAR, OAK) and three open-label, single arm studies (PCD4989g, BIRCH, FIR) which enrolled 1636 patients with metastatic NSCLC, and 980 patients with other tumor types. TECENTRIQ was administered at a dose of 1200 mg intravenously every 3 weeks in all studies except PCD4989g. Among the 2616 patients who received a single-agent TECENTRIQ, 36% were exposed for longer than 6 months and 20% were exposed for longer than 12 months. Using the dataset described for patients who received TECENTRIQ as a single-agent, the most common adverse reactions in ≥ 20% of patients were fatigue/asthenia (48%), decreased appetite (25%), nausea (24%), cough (22%), and dyspnea (22%). In addition, the data reflect exposure to TECENTRIQ as a single agent as adjuvant therapy in 495 patients with early stage NSCLC enrolled in a randomized study (IMpower010).
In addition, the data reflect exposure to TECENTRIQ in combination with other antineoplastic drugs in 2421 patients with NSCLC (N = 2223) or SCLC (N = 198) enrolled in five randomized, active-controlled trials, including IMpower150, IMpower130 and IMpower133. Among the 2421 patients, 53% were exposed to TECENTRIQ for longer than 6 months and 29% were exposed to TECENTRIQ for longer than 12 months. Among the 2421 patients with NSCLC and SCLC who received TECENTRIQ in combination with other antineoplastic drugs, the most common adverse reactions in ≥20% of patients were fatigue/asthenia (49%), nausea (38%), alopecia (35%), constipation (29%), diarrhea (28%) and decreased appetite (27%).
The data also reflect exposure to TECENTRIQ administered in combination with cobimetinib and vemurafenib in 230 patients enrolled in IMspire150. Among the 230 patients, 62% were exposed to TECENTRIQ for longer than 6 months and 42% were exposed to TECENTRIQ for longer than 12 months.
Non-Small Cell Lung Cancer (NSCLC)
Adjuvant Treatment of Early-stage NSCLC
IMpower010
The safety of TECENTRIQ was evaluated in IMpower010, a multicenter, open-label, randomized trial for the adjuvant treatment of patients with stage IB (tumors ≥ 4 cm) - IIIA NSCLC who had complete tumor resection and received up to 4 cycles of cisplatin-based adjuvant chemotherapy. Patients received TECENTRIQ 1200 mg every 3 weeks (n=495) for 1 year (16 cycles), unless disease progression or unacceptable toxicity occurred, or best supportive care [see Clinical Studies (14.1)]. The median number of cycles received was 16 (range: 1, 16).
Fatal adverse reactions occurred in 1.8% of patients receiving TECENTRIQ; these included multiple organ dysfunction syndrome, pneumothorax, interstitial lung disease, arrhythmia, acute cardiac failure, myocarditis, cerebrovascular accident, death of unknown cause, and acute myeloid leukemia (1 patient each).
Serious adverse reactions occurred in 18% of patients receiving TECENTRIQ. The most frequent serious adverse reactions (>1%) were pneumonia (1.8%), pneumonitis (1.6%), and pyrexia (1.2%).
TECENTRIQ was discontinued due to adverse reactions in 18% of patients; the most common adverse reactions (≥1%) leading to TECENTRIQ discontinuation were pneumonitis (2.2%), hypothyroidism (1.6%), increased aspartate aminotranferase (1.4%), arthralgia (1.0%), and increased alanine aminotransferase (1.0%).
Adverse reactions leading to interruption of TECENTRIQ occurred in 29% of patients; the most common (>1%) were rash (3.0%), hyperthyroidism (2.8%), hypothyroidism (1.6%), increased AST (1.6%), pyrexia (1.6%), increased ALT (1.4%), upper respiratory tract infection (1.4%), headache (1.2%), peripheral neuropathy (1.2%), and pneumonia (1.2%).
Tables 4 and 5 summarize adverse reactions and selected laboratory abnormalities in patients receiving TECENTRIQ in IMpower010.
Table 4: Adverse Reactions Occurring in ≥10% of Patients with Early Stage NSCLC Receiving TECENTRIQ in IMpower010 Adverse Reaction* TECENTRIQ
N = 495Best Supportive Care
N = 495All Grades
(%)Grades 3–4
(%)All Grades
(%)Grades 3–4
(%)- *
- Graded per NCI CTCAE v4.0
- †
- Includes rash, dermatitis, genital rash, skin exfoliation, rash maculo-papular, rash erythematous, rash papular, lichen planus, eczema asteatotic, dermatitis exfoliative, palmar-plantar erythrodysaesthesia syndrome, dyshidrotic eczema, eczema, drug eruption, rash pruritic, toxic skin eruption, dermatitis acneiform
- ‡
- Includes hypothyroidism, autoimmune hypothyroidism, primary hypothyroidism, blood thyroid stimulating hormone increased
- §
- Productive cough, upper airway cough syndrome, cough
- ¶
- Includes pyrexia, body temperature increased, hyperthermia
- #
- Includes fatigue, asthenia
- Þ
- Includes paraesthesia, neuropathy peripheral, peripheral sensory neuropathy, hypoaesthesia, polyneuropathy, dysaesthesia, neuralgia, axonal neuropathy
- ß
- Includes myalgia, bone pain, back pain, spinal pain, musculoskeletal chest pain, pain in extremity, neck pain, non-cardiac chest pain, musculoskeletal discomfort, musculoskeletal stiffness, musculoskeletal pain
- à
- Includes arthralgia, arthritis
Skin and Subcutaneous Tissue Rash† 17 1.2 1.4 0 Pruritus 10 0 0.6 0 Endocrine Disorders Hypothyroidism‡ 14 0 0.6 0 Respiratory, Thoracic and Mediastinal Cough§ 16 0 11 0 General Pyrexia¶ 14 0.8 2.2 0.2 Fatigue# 14 0.6 5 0.2 Nervous System Disorders Peripheral neuropathyÞ 12 0.4 7 0.2 Musculoskeletal and Connective Tissue Musculoskeletal painß 14 0.8 9 0.2 Arthralgiaà 11 0.6 6 0 Table 5: Laboratory Abnormalities Worsening from Baseline Occurring in ≥20% of Patients with Early Stage NSCLC Receiving TECENTRIQ in IMpower010 Laboratory Abnormality* TECENTRIQ† Best Supportive Care† All Grades
(%)Grades 3–4
(%)All Grades
(%)Grades 3–4
(%)- *
- Graded per NCI CTCAE v4.0, except for increased creatinine which only includes patients with creatinine increase based on upper limit of normal definition for Grade 1 events (NCI CTCAE v5.0).
- †
- The denominators used to calculate the rate varied from 78-480 for BSC arm and 483 for atezolizumab are for all tests of interest based on the number of patients with a baseline value and at least one post-treatment value.
Chemistry Increased aspartate aminotransferase 34 2.5 18 0 Increased alanine aminotransferase 30 3.3 19 0.4 Hyperkalemia 24 3.5 15 2.5 Increased blood creatinine 31 0.2 23 0.2 Metastatic Chemotherapy-Naïve NSCLC
IMpower110
The safety of TECENTRIQ was evaluated in IMpower110, a multicenter, international, randomized, open-label study in 549 chemotherapy-naïve patients with stage IV NSCLC, including those with EGFR or ALK genomic tumor aberrations. Patients received TECENTRIQ 1200 mg every 3 weeks (n=286) or platinum-based chemotherapy consisting of carboplatin or cisplatin with either pemetrexed or gemcitabine (n=263) until disease progression or unacceptable toxicity [see Clinical Studies (14.1)]. IMpower110 enrolled patients whose tumors express PD-L1 (PD-L1 stained ≥ 1% of tumor cells [TC] or PD-L1 stained tumor-infiltrating immune cells [IC] covering ≥ 1% of the tumor area). The median duration of exposure to TECENTRIQ was 5.3 months (0 to 33 months).
Fatal adverse reactions occurred in 3.8% of patients receiving TECENTRIQ; these included death (reported as unexplained death and death of unknown cause), aspiration, chronic obstructive pulmonary disease, pulmonary embolism, acute myocardial infarction, cardiac arrest, mechanical ileus, sepsis, cerebral infarction, and device occlusion (1 patient each).
Serious adverse reactions occurred in 28% of patients receiving TECENTRIQ. The most frequent serious adverse reactions (>2%) were pneumonia (2.8%), chronic obstructive pulmonary disease (2.1%) and pneumonitis (2.1%).
TECENTRIQ was discontinued due to adverse reactions in 6% of patients; the most common adverse reactions (≥2 patients) leading to TECENTRIQ discontinuation were peripheral neuropathy and pneumonitis.
Adverse reactions leading to interruption of TECENTRIQ occurred in 26% of patients; the most common (>1%) were ALT increased (2.1%), AST increased (2.1%), pneumonitis (2.1%), pyrexia (1.4%), pneumonia (1.4%) and upper respiratory tract infection (1.4%).
Tables 6 and 7 summarize adverse reactions and selected laboratory abnormalities in patients receiving TECENTRIQ in IMpower110.
Table 6: Adverse Reactions Occurring in ≥10% of Patients with NSCLC Receiving TECENTRIQ in IMpower110 Adverse Reaction TECENTRIQ
N = 286Platinum-Based Chemotherapy
N = 263All Grades
(%)Grades 3–4
(%)All Grades
(%)Grades 3–4
(%)Graded per NCI CTCAE v4.0 Gastrointestinal Nausea 14 0.3 34 1.9 Constipation 12 1.0 22 0.8 Diarrhea 11 0 12 0.8 General Fatigue/asthenia 25 1.4 34 4.2 Pyrexia 14 0 9 0.4 Metabolism and Nutrition Decreased appetite 15 0.7 19 0 Respiratory, Thoracic and Mediastinal Dyspnea 14 0.7 10 0 Cough 12 0.3 10 0 Table 7: Laboratory Abnormalities Worsening from Baseline Occurring in ≥20% of Patients Receiving TECENTRIQ in IMpower110 Laboratory Abnormality TECENTRIQ Platinum-Based Chemotherapy All Grades
(%)Grades 3–4
(%)All Grades
(%)Grades 3–4
(%)Each test incidence is based on the number of patients who had at least one on-study laboratory measurement available: TECENTRIQ (range: 278-281); platinum-based chemotherapy (range: 256-260). Graded per NCI CTCAE v4.0. Increased blood creatinine only includes patients with test results above the normal range. Hematology Anemia 69 1.8 94 20 Lymphopenia 47 9 59 17 Chemistry Hypoalbuminemia 48 0.4 39 2 Increased alkaline phosphatase 46 2.5 42 1.2 Hyponatremia 44 9 36 7 Increased ALT 38 3.2 32 0.8 Increased AST 36 3.2 32 0.8 Hyperkalemia 29 3.9 36 2.7 Hypocalcemia 24 1.4 24 2.7 Increased blood creatinine 24 0.7 33 1.5 Hypophosphatemia 23 3.6 21 2 IMpower150
The safety of TECENTRIQ with bevacizumab, paclitaxel and carboplatin was evaluated in IMpower150, a multicenter, international, randomized, open-label trial in which 393 chemotherapy-naïve patients with metastatic non-squamous NSCLC received TECENTRIQ 1200 mg with bevacizumab 15 mg/kg, paclitaxel 175 mg/m2 or 200 mg/m2, and carboplatin AUC 6 mg/mL/min intravenously every 3 weeks for a maximum of 4 or 6 cycles, followed by TECENTRIQ 1200 mg with bevacizumab 15 mg/kg intravenously every 3 weeks until disease progression or unacceptable toxicity [see Clinical Studies (14.1)]. The median duration of exposure to TECENTRIQ was 8.3 months in patients receiving TECENTRIQ with bevacizumab, paclitaxel, and carboplatin.
Fatal adverse reactions occurred in 6% of patients receiving TECENTRIQ; these included hemoptysis, febrile neutropenia, pulmonary embolism, pulmonary hemorrhage, death, cardiac arrest, cerebrovascular accident, pneumonia, aspiration pneumonia, chronic obstructive pulmonary disease, intracranial hemorrhage, intestinal angina, intestinal ischemia, intestinal obstruction and aortic dissection.
Serious adverse reactions occurred in 44%. The most frequent serious adverse reactions (>2%) were febrile neutropenia, pneumonia, diarrhea, and hemoptysis.
TECENTRIQ was discontinued due to adverse reactions in 15% of patients; the most common adverse reaction leading to discontinuation was pneumonitis (1.8%).
Adverse reactions leading to interruption of TECENTRIQ occurred in 48%; the most common (>1%) were neutropenia, thrombocytopenia, fatigue/asthenia, diarrhea, hypothyroidism, anemia, pneumonia, pyrexia, hyperthyroidism, febrile neutropenia, increased ALT, dyspnea, dehydration and proteinuria.
Tables 8 and 9 summarize adverse reactions and laboratory abnormalities in patients receiving TECENTRIQ with bevacizumab, paclitaxel, and carboplatin in IMpower150.
Table 8: Adverse Reactions Occurring in ≥15% of Patients with NSCLC Receiving TECENTRIQ in IMpower150 Adverse Reaction TECENTRIQ with Bevacizumab, Paclitaxel, and Carboplatin
N = 393Bevacizumab, Paclitaxel and Carboplatin
N = 394All Grades
(%)Grades 3–4
(%)All Grades
(%)Grades 3–4
(%)Graded per NCI CTCAE v4.0 - *
- Includes neuropathy peripheral, peripheral sensory neuropathy, hypoesthesia, paraesthesia, dysesthesia, polyneuropathy
- †
- Includes rash, rash maculo-papular, drug eruption, eczema, eczema asteatotic, dermatitis, contact dermatitis, rash erythematous, rash macular, pruritic rash, seborrheic dermatitis, dermatitis psoriasiform
- ‡
- Includes pain in extremity, musculoskeletal chest pain, musculoskeletal discomfort, neck pain, back pain, myalgia, and bone pain
- §
- Includes diarrhea, gastroenteritis, colitis, enterocolitis
- ¶
- Data based on Preferred Terms since laboratory data for proteinuria were not systematically collected
Nervous System Neuropathy* 56 3 47 3 Headache 16 0.8 13 0 General Fatigue/Asthenia 50 6 46 6 Pyrexia 19 0.3 9 0.5 Skin and Subcutaneous Tissue Alopecia 48 0 46 0 Rash† 23 2 10 0.3 Musculoskeletal and Connective Tissue Myalgia/Pain‡ 42 3 34 2 Arthralgia 26 1 22 1 Gastrointestinal Nausea 39 4 32 2 Diarrhea§ 33 6 25 0.5 Constipation 30 0.3 23 0.3 Vomiting 19 2 18 1 Metabolism and Nutrition Decreased appetite 29 4 21 0.8 Vascular Hypertension 25 9 22 8 Respiratory Cough 20 0.8 19 0.3 Epistaxis 17 1 22 0.3 Renal Proteinuria¶ 16 3 15 3 Table 9: Laboratory Abnormalities Worsening from Baseline Occurring in ≥20% of Patients with NSCLC Receiving TECENTRIQ in IMpower150 Laboratory Abnormality TECENTRIQ with Bevacizumab, Paclitaxel, and Carboplatin Bevacizumab, Paclitaxel and Carboplatin All Grades
(%)Grades 3–4
(%)All Grades
(%)Grades 3–4
(%)Each test incidence is based on the number of patients who had both baseline and at least one on-study laboratory measurement available: TECENTRIQ with bevacizumab, paclitaxel, and carboplatin range: 337-380); bevacizumab, paclitaxel, and carboplatin (range: 337-382). Graded per NCI CTCAE v4.0 - *
- NA = Not applicable. NCI CTCAE does not provide a Grades 3-4 definition for these laboratory abnormalities
Hematology Anemia 83 10 83 9 Neutropenia 52 31 45 26 Lymphopenia 48 17 38 13 Chemistry Hyperglycemia 61 0 60 0 Increased BUN 52 NA* 44 NA* Hypomagnesemia 42 2 36 1 Hypoalbuminemia 40 3 31 2 Increased AST 40 4 28 0.8 Hyponatremia 38 10 36 9 Increased Alkaline Phosphatase 37 2 32 1 Increased ALT 37 6 28 0.5 Increased TSH 30 NA* 20 NA* Hyperkalemia 28 3 25 2 Increased Creatinine 28 1 19 2 Hypocalcemia 26 3 21 3 Hypophosphatemia 25 4 18 4 Hypokalemia 23 7 14 4 Hyperphosphatemia 25 NA* 19 NA* IMpower130
The safety of TECENTRIQ with paclitaxel protein-bound and carboplatin was evaluated in IMpower130, a multicenter, international, randomized, open-label trial in which 473 chemotherapy-naïve patients with metastatic non-squamous NSCLC received TECENTRIQ 1200 mg and carboplatin AUC 6 mg/mL/min intravenously on Day 1 and paclitaxel protein-bound 100 mg/m2 intravenously on Day 1, 8, and 15 of each 21-day cycle for a maximum of 4 or 6 cycles, followed by TECENTRIQ 1200 mg intravenously every 3 weeks until disease progression or unacceptable toxicity [see Clinical Studies (14.1)]. Among patients receiving TECENTRIQ, 55% were exposed for 6 months or longer and 3.5% were exposed for greater than one year.
Fatal adverse reactions occurred in 5.3% of patients receiving TECENTRIQ; these included pneumonia (1.1%), pulmonary embolism (0.8%), myocardial infarction (0.6%), cardiac arrest (0.4%), pneumonitis (0.4%) and sepsis, septic shock, staphylococcal sepsis, aspiration, respiratory distress, cardiorespiratory arrest, ventricular tachycardia, death (not otherwise specified), and hepatic cirrhosis (0.2% each).
Serious adverse reactions occurred in 51% of patients receiving TECENTRIQ. The most frequent serious adverse reactions (≥2%) were pneumonia (6%), diarrhea (3%), lung infection (3%), pulmonary embolism (3%), chronic obstructive pulmonary disease exacerbation (2.5%), dyspnea (2.3%), and febrile neutropenia (1.9%).
TECENTRIQ was discontinued due to adverse reactions in 13% of patients; the most common adverse reactions leading to discontinuation were pneumonia (0.8%), pulmonary embolism (0.8%), fatigue (0.6%), dyspnea (0.6%), pneumonitis (0.6%), neutropenia (0.4%), nausea (0.4%), renal failure (0.4%), cardiac arrest (0.4%), and septic shock (0.4%).
Adverse reactions leading to interruption of TECENTRIQ occurred in 62% of patients; the most common (>1%) were neutropenia, thrombocytopenia, anemia, diarrhea, fatigue/asthenia, pneumonia, dyspnea, pneumonitis, pyrexia, nausea, acute kidney injury, vomiting, pulmonary embolism, arthralgia, infusion-related reaction, abdominal pain, chronic obstructive pulmonary disease exacerbation, dehydration, and hypokalemia.
Tables 10 and 11 summarize adverse reactions and laboratory abnormalities in patients receiving TECENTRIQ with paclitaxel protein-bound and carboplatin in IMpower130.
Table 10: Adverse Reactions Occurring in ≥20% of Patients with NSCLC Receiving TECENTRIQ in IMpower130 Adverse Reaction TECENTRIQ with Paclitaxel Protein-Bound and Carboplatin
N = 473Paclitaxel Protein-Bound and Carboplatin
N = 232All Grades (%) Grades 3–4 (%) All Grades (%) Grades 3–4 (%) Graded per NCI CTCAE v4.0 - *
- Includes diarrhea, colitis, and gastroenteritis
- †
- Includes back pain, pain in extremity, myalgia, musculoskeletal chest pain, bone pain, neck pain and musculoskeletal discomfort
- ‡
- Includes neuropathy peripheral, peripheral sensory neuropathy, hypoesthesia, paresthesia, dysesthesia, polyneuropathy
- §
- Includes dyspnea, dyspnea exertional and wheezing
- ¶
- Includes rash, rash maculo-papular, eczema, rash pruritic, rash erythematous, dermatitis, dermatitis contact, drug eruption, seborrheic dermatitis and rash macular.
General Fatigue/Asthenia 61 11 60 8 Gastrointestinal Nausea 50 3.4 46 2.2 Diarrhea * 43 6 32 6 Constipation 36 1.1 31 0 Vomiting 27 2.7 19 2.2 Musculoskeletal and Connective Tissue Myalgia/Pain † 38 3 22 0.4 Nervous System Neuropathy ‡ 33 2.5 28 2.2 Respiratory, Thoracic and Mediastinal Dyspnea § 32 4.9 25 1.3 Cough 27 0.6 17 0 Skin and Subcutaneous Tissue Alopecia 32 0 27 0 Rash ¶ 20 0.6 11 0.9 Metabolism and Nutrition Decreased appetite 30 2.1 26 2.2 Table 11: Laboratory Abnormalities Worsening from Baseline Occurring in ≥20% of Patients Receiving TECENTRIQ in IMpower130 Laboratory Abnormality TECENTRIQ with Paclitaxel Protein-Bound and Carboplatin
N = 473Paclitaxel Protein-Bound
and Carboplatin
N = 232All Grades
(%)Grades 3–4
(%)All Grades
(%)Grades 3–4 (%) Each test incidence is based on the number of patients who had both baseline and at least one on-study laboratory measurement available: TECENTRIQ with paclitaxel protein-bound and carboplatin (range: 423 - 467); paclitaxel protein-bound and carboplatin (range: 218 - 229). Graded per NCI CTCAE v4.0. - *
- NA = Not applicable. NCI CTCAE does not provide a Grades 3-4 definition for these laboratory abnormalities
Hematology Anemia 92 33 87 25 Neutropenia 75 50 67 39 Thrombocytopenia 73 19 59 13 Lymphopenia 71 23 61 16 Chemistry Hyperglycemia 75 8 66 8 Hypomagnesemia 50 3.4 42 3.2 Hyponatremia 37 9 28 7 Hypoalbuminemia 35 1.3 31 0 Increased ALT 31 2.8 24 3.9 Hypocalcemia 31 2.6 27 1.8 Hypophosphatemia 29 6 20 3.2 Increased AST 28 2.2 24 1.8 Increased TSH 26 NA* 5 NA* Hypokalemia 26 6 24 4.4 Increased Alkaline Phosphatase 25 2.6 22 1.3 Increased Blood Creatinine 23 2.8 16 0.4 Hyperphosphatemia 21 NA* 13 NA* Previously Treated Metastatic NSCLC
The safety of TECENTRIQ was evaluated in OAK, a multicenter, international, randomized, open-label trial in patients with metastatic NSCLC who progressed during or following a platinum-containing regimen, regardless of PD-L1 expression [see Clinical Studies (14.1)]. A total of 609 patients received TECENTRIQ 1200 mg intravenously every 3 weeks until unacceptable toxicity, radiographic progression, or clinical progression or docetaxel (n=578) 75 mg/m2 intravenously every 3 weeks until unacceptable toxicity or disease progression. The study excluded patients with active or prior autoimmune disease or with medical conditions that required systemic corticosteroids. The median duration of exposure was 3.4 months (0 to 26 months) in TECENTRIQ-treated patients and 2.1 months (0 to 23 months) in docetaxel-treated patients.
The study population characteristics were: median age of 63 years (25 to 85 years), 46% age 65 years or older, 62% male, 71% White, 20% Asian, 68% former smoker, 16% current smoker, and 63% had ECOG performance status of 1.
Fatal adverse reactions occurred in 1.6% of patients; these included pneumonia, sepsis, septic shock, dyspnea, pulmonary hemorrhage, sudden death, myocardial ischemia or renal failure.
Serious adverse reactions occurred in 33.5% of patients. The most frequent serious adverse reactions (>1%) were pneumonia, sepsis, dyspnea, pleural effusion, pulmonary embolism, pyrexia and respiratory tract infection.
TECENTRIQ was discontinued due to adverse reactions in 8% of patients. The most common adverse reactions leading to TECENTRIQ discontinuation were fatigue, infections and dyspnea. Adverse reactions leading to interruption of TECENTRIQ occurred in 25% of patients; the most common (>1%) were pneumonia, liver function test abnormality, dyspnea, fatigue, pyrexia, and back pain.
Tables 12 and 13 summarize adverse reactions and laboratory abnormalities, respectively, in OAK.
Table 12: Adverse Reactions Occurring in ≥10% of Patients with NSCLC Receiving TECENTRIQ in OAK Adverse Reaction TECENTRIQ
N = 609Docetaxel
N = 578All Grades
(%)Grades 3–4
(%)All Grades
(%)Grades 3–4
(%)Graded per NCI CTCAE v4.0 General Fatigue/Asthenia* 44 4 53 6 Pyrexia 18 <1 13 <1 Respiratory Cough† 26 <1 21 <1 Dyspnea 22 2.8 21 2.6 Metabolism and Nutrition Decreased appetite 23 <1 24 1.6 Musculoskeletal Myalgia/Pain‡ 20 1.3 20 <1 Arthralgia 12 0.5 10 0.2 Gastrointestinal Nausea 18 <1 23 <1 Constipation 18 <1 14 <1 Diarrhea 16 <1 24 2 Skin Rash§ 12 <1 10 0 Table 13: Laboratory Abnormalities Worsening from Baseline Occurring in ≥20% of Patients with NSCLC Receiving TECENTRIQ in OAK Laboratory Abnormality TECENTRIQ Docetaxel All Grades
(%)Grades 3–4
(%)All Grades
(%)Grades 3–4
(%)Each test incidence is based on the number of patients who had both baseline and at least one on-study laboratory measurement available: TECENTRIQ (range: 546–585) and docetaxel (range: 532–560). Graded according to NCI CTCAE version 4.0 Hematology Anemia 67 3 82 7 Lymphocytopenia 49 14 60 21 Chemistry Hypoalbuminemia 48 4 50 3 Hyponatremia 42 7 31 6 Increased Alkaline Phosphatase 39 2 25 1 Increased AST 31 3 16 0.5 Increased ALT 27 3 14 0.5 Hypophosphatemia 27 5 23 4 Hypomagnesemia 26 1 21 1 Increased Creatinine 23 2 16 1 Small Cell Lung Cancer (SCLC)
The safety of TECENTRIQ with carboplatin and etoposide was evaluated in IMpower133, a randomized, multicenter, double-blind, placebo-controlled trial in which 198 patients with ES-SCLC received TECENTRIQ 1200 mg and carboplatin AUC 5 mg/mL/min on Day 1 and etoposide 100 mg/m2 intravenously on Days 1, 2 and 3 of each 21-day cycle for a maximum of 4 cycles, followed by TECENTRIQ 1200 mg every 3 weeks until disease progression or unacceptable toxicity [see Clinical Studies (14.2)]. Among 198 patients receiving TECENTRIQ, 32% were exposed for 6 months or longer and 12% were exposed for 12 months or longer.
Fatal adverse reactions occurred in 2% of patients receiving TECENTRIQ. These included pneumonia, respiratory failure, neutropenia, and death (1 patient each).
Serious adverse reactions occurred in 37% of patients receiving TECENTRIQ. Serious adverse reactions in >2% were pneumonia (4.5%), neutropenia (3.5%), febrile neutropenia (2.5%), and thrombocytopenia (2.5%).
TECENTRIQ was discontinued due to adverse reactions in 11% of patients. The most frequent adverse reaction requiring permanent discontinuation in >2% of patients was infusion-related reactions (2.5%).
Adverse reactions leading to interruption of TECENTRIQ occurred in 59% of patients; the most common (>1%) were neutropenia (22%), anemia (9%), leukopenia (7%), thrombocytopenia (5%), fatigue (4.0%), infusion-related reaction (3.5%), pneumonia (2.0%), febrile neutropenia (1.5%), increased ALT (1.5%), and nausea (1.5%).
Tables 14 and 15 summarize adverse reactions and laboratory abnormalities, respectively, in patients who received TECENTRIQ with carboplatin and etoposide in IMpower133.
Table 14: Adverse Reactions Occurring in ≥20% of Patients with SCLC Receiving TECENTRIQ in IMpower133 Adverse Reaction TECENTRIQ with Carboplatin and Etoposide
N = 198Placebo with Carboplatin and Etoposide
N = 196All Grades
(%)Grades 3–4
(%)All Grades
(%)Grades 3–4
(%)Graded per NCI CTCAE v4.0 General Fatigue/asthenia 39 5 33 3 Gastrointestinal Nausea 38 1 33 1 Constipation 26 1 30 1 Vomiting 20 2 17 3 Skin and Subcutaneous Tissue Alopecia 37 0 35 0 Metabolism and Nutrition Decreased appetite 27 1 18 0 Table 15: Laboratory Abnormalities Worsening from Baseline Occurring in ≥20% of Patients with SCLC Receiving TECENTRIQ in IMpower133 Laboratory Abnormality TECENTRIQ with Carboplatin and Etoposide Placebo with Carboplatin and Etoposide All Grades
(%)Grades 3–4
(%)All Grades
(%)Grades 3–4
(%)Each test incidence is based on the number of patients who had both baseline and at least one on-study laboratory measurement available: TECENTRIQ (range: 181-193); Placebo (range: 181-196). Graded per NCI CTCAE v4.0 Hematology Anemia 94 17 93 19 Neutropenia 73 45 76 48 Thrombocytopenia 58 20 53 17 Lymphopenia 46 14 38 11 Chemistry Hyperglycemia 67 10 65 8 Increased Alkaline Phosphatase 38 1 35 2 Hyponatremia 34 15 33 11 Hypoalbuminemia 32 1 30 0 Decreased TSH* 28 NA† 15 NA† Hypomagnesemia 31 5 35 6 Hypocalcemia 26 3 28 5 Increased ALT 26 3 31 1 Increased AST 22 1 21 2 Increased Blood Creatinine 22 4 15 1 Hyperphosphatemia 21 NA† 23 NA† Increased TSH* 21 NA† 7 NA† Hepatocellular Carcinoma (HCC)
The safety of TECENTRIQ in combination with bevacizumab was evaluated in IMbrave150, a multicenter, international, randomized, open-label trial in patients with locally advanced or metastatic or unresectable hepatocellular carcinoma who have not received prior systemic treatment [see Clinical Studies (14.3)]. Patients received 1,200 mg of TECENTRIQ intravenously followed by 15 mg/kg bevacizumab (n=329) every 3 weeks, or 400 mg of sorafenib (n=156) given orally twice daily, until disease progression or unacceptable toxicity. The median duration of exposure to TECENTRIQ was 7.4 months (range: 0-16 months) and to bevacizumab was 6.9 months (range: 0-16 months).
Fatal adverse reactions occurred in 4.6% of patients in the TECENTRIQ and bevacizumab arm. The most common adverse reactions leading to death were gastrointestinal and esophageal varices hemorrhage (1.2%) and infections (1.2%).
Serious adverse reactions occurred in 38% of patients in the TECENTRIQ and bevacizumab arm. The most frequent serious adverse reactions (≥ 2%) were gastrointestinal hemorrhage (7%), infections (6%), and pyrexia (2.1%).
Adverse reactions leading to discontinuation of TECENTRIQ occurred in 9% of patients in the TECENTRIQ and bevacizumab arm. The most common adverse reactions leading to TECENTRIQ discontinuation were hemorrhages (1.2%), including gastrointestinal, subarachnoid, and pulmonary hemorrhages; increased transaminases or bilirubin (1.2%); infusion-related reaction/cytokine release syndrome (0.9%); and autoimmune hepatitis (0.6%).
Adverse reactions leading to interruption of TECENTRIQ occurred in 41% of patients in the TECENTRIQ and bevacizumab arm; the most common (≥ 2%) were liver function laboratory abnormalities including increased transaminases, bilirubin, or alkaline phosphatase (8%); infections (6%); gastrointestinal hemorrhages (3.6%); thrombocytopenia/decreased platelet count (3.6%); hyperthyroidism (2.7%); and pyrexia (2.1%).
Immune-related adverse reactions requiring systemic corticosteroid therapy occurred in 12% of patients in the TECENTRIQ and bevacizumab arm.
Tables 16 and 17 summarize adverse reactions and laboratory abnormalities, respectively, in patients who received TECENTRIQ and bevacizumab in IMbrave150.
Table 16: Adverse Reactions Occurring in ≥10% of Patients with HCC Receiving TECENTRIQ in IMbrave150 Adverse Reaction TECENTRIQ in combination with Bevacizumab
(n = 329)Sorafenib
(n=156)All Grades*
(%)Grades 3–4*
(%)All Grades*
(%)Grades 3–4*
(%)Vascular Disorders Hypertension 30 15 24 12 General Disorders and Administration Site Conditions Fatigue/asthenia† 26 2 32 6 Pyrexia 18 0 10 0 Renal and Urinary Disorders Proteinuria 20 3 7 0.6 Investigations Weight Decreased 11 0 10 0 Skin and Subcutaneous Tissue Disorders Pruritus 19 0 10 0 Rash 12 0 17 2.6 Gastrointestinal Disorders Diarrhea 19 1.8 49 5 Constipation 13 0 14 0 Abdominal Pain 12 0 17 0 Nausea 12 0 16 0 Vomiting 10 0 8 0 Metabolism and Nutrition Disorders Decreased Appetite 18 1.2 24 3.8 Respiratory, Thoracic and Mediastinal Disorders Cough 12 0 10 0 Epistaxis 10 0 4.5 0 Injury, Poisoning and Procedural Complications Infusion-Related Reaction 11 2.4 0 0 Table 17: Laboratory Abnormalities Worsening from Baseline Occurring in ≥20% of Patients with HCC Receiving TECENTRIQ in IMbrave150 Laboratory Abnormality TECENTRIQ in combination with Bevacizumab
(n = 329)Sorafenib
(n=156)All Grades*
(%)Grades 3–4*
(%)All Grades*
(%)Grades 3–4*
(%)Each test incidence is based on the number of patients who had both baseline and at least one on-study laboratory measurement available: TECENTRIQ plus bevacizumab (222-323) and sorafenib (90-153) - *
- Graded per NCI CTCAE v4.0
Chemistry Increased AST 86 16 90 16 Increased Alkaline Phosphatase 70 4 76 4.6 Increased ALT 62 8 70 4.6 Decreased Albumin 60 1.5 54 0.7 Decreased Sodium 54 13 49 9 Increased Glucose 48 9 43 4.6 Decreased Calcium 30 0.3 35 1.3 Decreased Phosphorus 26 4.7 58 16 Increased Potassium 23 1.9 16 2 Hypomagnesemia 22 0 22 0 Hematology Decreased Platelet 68 7 63 4.6 Decreased Lymphocytes 62 13 58 11 Decreased Hemoglobin 58 3.1 62 3.9 Increased Bilirubin 57 8 59 14 Decreased Leukocyte 32 3.4 29 1.3 Decreased Neutrophil 23 2.3 16 1.1 Melanoma
The safety of TECENTRIQ, administered with cobimetinib and vemurafenib was evaluated in IMspire150, a double-blind, randomized (1:1), placebo-controlled study conducted in patients with previously untreated BRAF V600 mutation-positive metastatic or unresectable melanoma [see Clinical Studies (14.4)]. Patients received TECENTRIQ with cobimetinib and vemurafenib (N=230) or placebo with cobimetinib and vemurafenib (n=281).
Among the 230 patients who received TECENTRIQ administered with cobimetinib and vemurafenib, the median duration of exposure to TECENTRIQ was 9.2 months (range: 0-30 months) to cobimetinib was 10.0 months (range: 1-31 months) and to vemurafenib was 9.8 months (range: 1-31 months).
Fatal adverse reactions occurred in 3% of patients in the TECENTRIQ plus cobimetinib and vemurafenib arm. Adverse reactions leading to death were hepatic failure, fulminant hepatitis, sepsis, septic shock, pneumonia, and cardiac arrest.
Serious adverse reactions occurred in 45% of patients in the TECENTRIQ plus cobimetinib and vemurafenib arm. The most frequent (≥ 2%) serious adverse reactions were hepatotoxicity (7%), pyrexia (6%), pneumonia (4.3%), malignant neoplasms (2.2%), and acute kidney injury (2.2%).
Adverse reactions leading to discontinuation of TECENTRIQ occurred in 21% of patients in the TECENTRIQ plus cobimetinib and vemurafenib arm. The most frequent (≥ 2%) adverse reactions leading to TECENTRIQ discontinuation were increased ALT (2.2%) and pneumonitis (2.6%).
Adverse reactions leading to interruption of TECENTRIQ occurred in 68% of patients in the TECENTRIQ plus cobimetinib and vemurafenib arm. The most frequent (≥ 2%) adverse reactions leading to TECENTRIQ interruption were pyrexia (14%), increased ALT (13%), hyperthyroidism (10%), increased AST (10%), increased lipase (9%), increased amylase (7%), pneumonitis (5%), increased CPK (4.3%), diarrhea (3.5%), pneumonia (3.5%), asthenia (3%), rash (3%), influenza (3%), arthralgia (2.6%), fatigue (2.2%), dyspnea (2.2%), cough (2.2%), peripheral edema (2.2%), uveitis (2.2%), bronchitis (2.2%), hypothyroidism (2.2%), and respiratory tract infection (2.2%).
Tables 18 and 19 summarize the incidence of adverse reactions and laboratory abnormalities in Study IMspire150.
Table 18: Adverse Reactions Occurring in ≥10% of Patients on the TECENTRIQ plus Cobimetinib and Vemurafenib Arm or the Placebo plus Cobimetinib and Vemurafenib Arm and at a Higher Incidence (Between Arm Difference of ≥ 5% All Grades or ≥ 2% Grades 3-4 TECENTRIQ in IMspire150) Adverse Reaction TECENTRIQ in combination with Cobimetinib and Vemurafenib
(n=230)Placebo with Cobimetinib and Vemurafenib
(n=281)All Grades
(%)Grade 3–4
(%)All Grades
(%)Grade 3–4
(%)- *
- Includes rash, rash maculo-papular, dermatitis acneiform, rash macular, rash erythematous, eczema, skin exfoliation, rash papular, rash pustular, palmar-plantar erythrodysaesthesia syndrome, dermatitis, dermatitis contact, erythema multiforme, rash pruritic, drug eruption, nodular rash, dermatitis allergic, exfoliative rash, dermatitis exfoliative generalised and rash morbilliform
- †
- Includes fatigue, asthenia and malaise
- ‡
- Includes pyrexia and hyperpyrexia
- §
- Includes edema peripheral, lymphoedema, oedema, face oedema, eyelid oedema, periorbital oedema, lip oedema and generalised oedema
- ¶
- Includes alanine aminotransferase increased, aspartate aminotransferase increased, blood bilirubin increased, transaminases increased, hepatitis, hepatic enzyme increased, hepatotoxicity, hypertransaminasaemia, bilirubin conjugated increased, hepatocellular injury, hyperbilirubinaemia, liver function test increased, hepatic failure, hepatitis fulminant and liver function test abnormal
- #
- Includes stomatitis, mucosal inflammation, aphthous ulcer, mouth ulceration, cheilitis and glossitis
- Þ
- Includes arthralgia, myalgia, pain in extremity, back pain, musculoskeletal pain, arthritis, neck pain, musculoskeletal chest pain, musculoskeletal stiffness, bone pain, spinal pain, immune-mediated arthritis, joint stiffness and non-cardiac chest pain
- ß
- Includes hypothyroidism and blood thyroid stimulating hormone increased
- à
- Includes infusion related reaction and hypersensitivity
- è
- Includes pneumonitis and interstitial lung disease
- ð
- Includes hypertension, blood pressure increased, hypertensive crisis
Skin and Subcutaneous Tissue Disorders Rash* 75 27 72 23 Pruritus 26 <1 17 <1 Photosensitivity reaction 21 <1 25 3.2 General Disorders and Administration Site Conditions Fatigue † 51 3 45 1.8 Pyrexia ‡ 49 1.7 35 2.1 Edema § 26 <1 21 0 Gastrointestinal Disorders Hepatotoxicity ¶ 50 21 36 13 Nausea 30 <1 32 2.5 Stomatitis # 23 1.3 15 <1 Musculoskeletal and Connective Tissue Disorders Musculoskeletal pain Þ 62 4.3 48 3.2 Endocrine Disorders Hypothyroidism ß 22 0 10 0 Hyperthyroidism 18 <1 8 0 Injury, Poisoning and Procedural Complications Infusion-related reaction à 10 2.6 8 <1 Respiratory, Thoracic and Mediastinal Disorders Pneumonitis è 12 1.3 6 <1 Vascular Disorders Hypertension ð 17 10 18 7 Clinically important adverse reactions in < 10% of patients who received TECENTRIQ plus cobimetinib and vemurafenib were:
Cardiac Disorders: Arrhythmias, ejection fraction decreased, electrocardiogram QT prolonged
Eye Disorders: Uveitis
Gastrointestinal disorders: Pancreatitis
Infections and infestations: Pneumonia, urinary tract infection
Metabolism and nutrition disorders: Hyperglycemia
Nervous system Disorders: Dizziness, dysgeusia, syncope
Respiratory, thoracic and mediastinal disorders: Dyspnea, oropharyngeal pain
Skin and Subcutaneous Tissue Disorders: Vitiligo
Table 19: Laboratory Abnormalities Worsening from Baseline Occurring in ≥ 20% of Patients Receiving TECENTRIQ Plus Cobimetinib and Vemurafenib Arm or the Placebo Plus Cobimetinib and Vemurafenib Arm and at a Higher Incidence (Between Arm Difference of ≥ 5% All Grades or ≥ 2% Grades 3-4) in IMspire150 Laboratory Abnormality TECENTRIQ in combination with Cobimetinib and Vemurafenib
(n=230)Placebo with Cobimetinib and Vemurafenib
(n=281)All Grades
(%)Grade 3–4
(%)All Grades
(%)Grade 3–4
(%)Graded per NCI CTCAE v4.0. Each test incidence is based on the number of patients who had both baseline and at least one on-study laboratory measurement available: TECENTRIQ plus cobimetinib and vemurafenib (28-277), placebo plus cobimetinib and vemurafenib arm (25-230). Hematology Decreased Lymphocytes 80 24 72 17 Decreased Hemoglobin 77 2.6 72 2.2 Decreased Platelet 34 1.3 24 0.4 Decreased Neutrophils 26 2.2 19 1.5 Chemistry Increased Creatine Kinase 88 22 81 18 Increased AST 80 13 68 6 Increased ALT 79 18 62 12 Increased Triacylglycerol Lipase 75 46 62 35 Increased Alkaline Phosphatase 73 6 63 2.9 Decreased Phosphorus 67 22 64 14 Increased Amylase 51 13 45 13 Increased Blood Urea Nitrogen 47 NA* 37 NA* Decreased Albumin 43 0.9 34 1.5 Increased Bilirubin 42 3.1 33 0.7 Decreased Calcium 41 1.3 28 0 Decreased Sodium 40 5 34 7 Decreased Thyroid-Stimulating Hormone 38 NA* 23 NA* Increased Thyroid-Stimulating Hormone † 37 NA* 33 NA* Decreased Potassium 36 5 22 4.3 Increased Triiodothyronine 33 NA* 18 NA* Increased Free Thyroxine 32 NA* 21 NA* Decreased Total Triiodothyronine 32 NA* 8 NA* Increased Potassium 29 1.3 19 1.4 Decreased Triiodothyronine 27 NA* 21 NA* Increased Sodium 20 0 13 0.4 Unresectable or Metastatic Alveolar Soft Part Sarcoma (ASPS)
The safety of TECENTRIQ was evaluated in 47 adult and 2 pediatric patients enrolled in Study ML39345 [see Clinical Studies (14.5)]. Adult patients received TECENTRIQ 1200 mg every 3 weeks and pediatric patients received 15 mg/kg up to a maximum 1200 mg every 3 weeks until disease progression or unacceptable toxicity. The median duration of exposure to TECENTRIQ was 8.9 months (1 to 40 months).
Serious adverse reactions occurred in 41% of patients receiving TECENTRIQ. The most frequent serious adverse reactions (>2%) were fatigue, pain in extremity, pulmonary hemorrhage, and pneumonia (4.1% each).
Dosage interruptions of TECENTRIQ due to an adverse reaction occurred in 35% of patients. The most common adverse reactions (≥3%) leading to dose interruptions were pneumonitis and pain in extremity (4.1% each).
Tables 20 and 21 summarize adverse reactions and laboratory abnormalities in Study ML39345.
Table 20: Adverse Reactions Occurring in ≥15% of Patients with ASPS Receiving TECENTRIQ in ML39345 Adverse Reaction TECENTRIQ
N = 49All Grades
(%)Grades 3–4
(%)Graded per NCI CTCAE v4.0 - *
- Includes abdominal pain and abdominal pain upper
- †
- Includes cough, upper-airway cough syndrome, and productive cough
- ‡
- Includes arthralgia, pain in extremity, myalgia, non-cardiac chest pain, neck pain, musculoskeletal chest pain, and back pain
- §
- Includes rash maculo-papular, rash, dermatitis acneiform, eczema, skin exfoliation, and drug eruption
- ¶
- Includes vertigo and dizziness
- #
- Includes pulmonary hemorrhage, hemoptysis, conjunctival hemorrhage, epistaxis, hematuria, rectal hemorrhage, and laryngeal hemorrhage
- Þ
- Includes atrial fibrillation, sinus bradycardia, ventricular tachycardia, and sinus tachycardia
- ß
- Includes hypothyroidism and blood thyroid stimulating hormone increased
General disorders and administration site conditions Fatigue 55 2 Pyrexia 25 2 Influenza like illness 18 0 Gastrointestinal disorders Nausea 43 0 Vomiting 37 0 Constipation 33 0 Diarrhea 27 2 Abdominal pain* 25 0 Metabolism and nutrition disorders Decreased appetite 22 2 Respiratory, Thoracic and Mediastinal Cough† 45 0 Dyspnea 33 0 Rhinitis allergic 16 0 Musculoskeletal and connective tissue disorders Musculoskeletal pain‡ 67 8 Skin and subcutaneous tissue disorders Rash§ 47 2 Nervous system disorders Headache 43 4 Dizziness¶ 29 4 Vascular disorders Hypertension 43 6 Hemorrhage# 29 2 Psychiatric disorders Insomnia 27 0 Anxiety 25 0 Cardiac Disorders ArrhythmiaÞ 22 2 Endocrine disoders Hypothyroidismß 25 0 Investigations Weight decreased 18 0 Weight increased 16 6 Table 21: Laboratory Abnormalities Worsening from Baseline Occurring in ≥20% of Patients with ASPS Receiving TECENTRIQ in ML39345 Laboratory Abnormality * TECENTRIQ† All Grades
(%)Grades 3–4
(%)- *
- Laboratory tests which do not have NCI CTCAE grading criteria are also included for All Grade assessments, which were performed by comparing to respective lab normal ranges.
- †
- The denominators used to calculate the rate varied from 4-49 for all tests of interest based on the number of patients with a baseline value and at least one on-study laboratory measurement available.
Hematology Decreased Hemoglobin 63 0 Decreased Platelets 27 0 Increased Platelets 29 0 Chemistry Increased Alkaline Phosphatase 29 0 Decreased Amylase 40 0 Increased Amylase 20 20 Decreased Bilirubin 49 0 Decreased Calcium 47 0 Increased Calcium 25 14 Decreased Glucose 33 0 Increased Glucose 78 0 Decreased Glucose (fasting) 25 0 Decreased Magnesium 21 0 Increased Magnesium 26 26 Increased AST 39 2 Increased ALT 33 2 Decreased Sodium 43 0 Increased Lipase 25 25 6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of TECENTRIQ. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
- Cardiac: pericarditis, pericardial effusion, cardiac tamponade
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Based on its mechanism of action [see Clinical Pharmacology (12.1)], TECENTRIQ can cause fetal harm when administered to a pregnant woman. There are no available data on the use of TECENTRIQ in pregnant women.
Animal studies have demonstrated that inhibition of the PD-L1/PD-1 pathway can lead to increased risk of immune-related rejection of the developing fetus resulting in fetal death (see Data). Advise females of reproductive potential of the potential risk to a fetus.
In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Data
Animal Data
Animal reproduction studies have not been conducted with TECENTRIQ to evaluate its effect on reproduction and fetal development. A literature-based assessment of the effects on reproduction demonstrated that a central function of the PD-L1/PD-1 pathway is to preserve pregnancy by maintaining maternal immune tolerance to a fetus. Blockage of PD-L1 signaling has been shown in murine models of pregnancy to disrupt tolerance to a fetus and to result in an increase in fetal loss; therefore, potential risks of administering TECENTRIQ during pregnancy include increased rates of abortion or stillbirth. As reported in the literature, there were no malformations related to the blockade of PD-L1/PD-1 signaling in the offspring of these animals; however, immune-mediated disorders occurred in PD-1 and PD-L1 knockout mice. Based on its mechanism of action, fetal exposure to atezolizumab may increase the risk of developing immune-mediated disorders or altering the normal immune response.
8.2 Lactation
Risk Summary
There is no information regarding the presence of atezolizumab in human milk, the effects on the breastfed infant, or the effects on milk production. As human IgG is excreted in human milk, the potential for absorption and harm to the infant is unknown. Because of the potential for serious adverse reactions in breastfed infants from TECENTRIQ, advise women not to breastfeed during treatment and for at least 5 months after the last dose.
8.3 Females and Males of Reproductive Potential
Pregnancy Testing
Verify pregnancy status in females of reproductive potential prior to initiating TECENTRIQ [see Use in Specific Populations (8.1)].
Contraception
Females
Based on its mechanism of action, TECENTRIQ can cause fetal harm when administered to a pregnant woman [see Use in Specific Populations (8.1)]. Advise females of reproductive potential to use effective contraception during treatment with TECENTRIQ and for at least 5 months following the last dose.
Infertility
Females
Based on animal studies, TECENTRIQ may impair fertility in females of reproductive potential while receiving treatment [see Nonclinical Toxicology (13.1)].
8.4 Pediatric Use
Alveolar Soft Part Sarcoma
The safety and effectiveness of TECENTRIQ for unresectable or metastatic ASPS have been established in pediatric patients aged 2 years and older. Use of TECENTRIQ for this indication is supported by evidence from an adequate and well controlled study of TECENTRIQ in adults and 2 adolescent pediatric patients (≥12 years of age) with ASPS with additional pharmacokinetic and safety data in pediatric patients 2 years to <17 years. These data suggest that atezolizumab exposure in pediatric patients aged 2 years and older is comparable with that of adults and is expected to result in similar safety and efficacy to that of adults [see Adverse Reactions (6.1), Pharmacokinetics (12.3), Clinical Studies (14.5)]. The course of unresectable or metastatic ASPS is sufficiently similar between pediatric patients 2 to 11 years old and that of adults and adolescent patients to allow extrapolation of efficacy and safety to pediatric patients 2 years and older.
The safety and effectiveness of TECENTRIQ for ASPS have not been established in pediatric patients younger than 2 years of age.
Solid Tumors and Lymphomas
The safety and effectiveness of TECENTRIQ in pediatric patients have not been established in non-small cell lung cancer, small-cell lung cancer, hepatocellular carcinoma, or melanoma.
The safety and effectiveness of TECENTRIQ were assessed, but not established in a single-arm, multi-center, multi-cohort trial (NCT02541604) in 60 pediatric patients aged 7 months to <17 years with relapsed or progressive solid tumors and lymphomas. No new safety signals were observed in pediatric patients in this study.
8.5 Geriatric Use
Of 2616 patients with metastatic NSCLC and other tumor types treated with single agent TECENTRIQ in clinical studies, 49% were 65 years and over and 15% were 75 years and over.
Of 2421 patients with NSCLC and SCLC treated with TECENTRIQ in combination with other antineoplastic drugs in clinical studies, 48% were 65 years and over and 10% were 75 years and over.
No overall differences in safety or effectiveness were observed between patients aged 65 years or older and younger patients.
-
11 DESCRIPTION
Atezolizumab is a programmed cell death ligand 1 (PD-L1) blocking antibody. Atezolizumab is an Fc-engineered, humanized, non-glycosylated IgG1 kappa immunoglobulin that has a calculated molecular mass of 145 kDa.
TECENTRIQ (atezolizumab) injection for intravenous use is a sterile, preservative-free, colorless to slightly yellow solution in single-dose vials. Each 20 mL vial contains 1200 mg of atezolizumab and is formulated in glacial acetic acid (16.5 mg), L-histidine (62 mg), polysorbate 20 (8 mg), and sucrose (821.6 mg), with a pH of 5.8. Each 14 mL vial contains 840 mg of atezolizumab and is formulated in glacial acetic acid (11.5 mg), L-histidine (43.4 mg), polysorbate 20 (5.6 mg), and sucrose (575.1 mg) with a pH of 5.8.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
PD L1 may be expressed on tumor cells and/or tumor infiltrating immune cells and can contribute to the inhibition of the anti-tumor immune response in the tumor microenvironment. Binding of PD L1 to the PD 1 and B7.1 receptors found on T cells and antigen presenting cells suppresses cytotoxic T-cell activity, T-cell proliferation and cytokine production.
Atezolizumab is a monoclonal antibody that binds to PD L1 and blocks its interactions with both PD 1 and B7.1 receptors. This releases the PD L1/PD 1 mediated inhibition of the immune response, including activation of the anti-tumor immune response without inducing antibody-dependent cellular cytotoxicity. In syngeneic mouse tumor models, blocking PD L1 activity resulted in decreased tumor growth.
In mouse models of cancer, dual inhibition of the PD-1/PD-L1 and MAPK pathways suppresses tumor growth and improves tumor immunogenicity through increased antigen presentation and T cell infiltration and activation compared to targeted therapy alone.
12.2 Pharmacodynamics
The exposure-response relationship and time course of pharmacodynamic response for the safety and effectiveness of atezolizumab have not been fully characterized.
12.3 Pharmacokinetics
Atezolizumab exposure increased dose proportionally over the dose range of 1 mg/kg to 20 mg/kg (0.07 to 1.33 times of the approved recommended doses), including a dose of 1200 mg administered every 3 weeks. Steady state was achieved after 6 to 9 weeks following multiple doses. The systemic accumulation ratio for every 2 weeks administration and every 3 weeks administration is 3.3- and 1.9- fold, respectively.
Elimination
The clearance (CV%) is 0.2 L/day (29%) and the terminal half-life is 27 days. Atezolizumab clearance was found to decrease over time, with a mean maximal reduction (CV%) from baseline value of 17% (41%); however, the decrease in clearance was not considered clinically relevant.
Specific Populations
The following factors had no clinically significant effect on the systemic exposure of atezolizumab: age (2 to 89 years), body weight, sex, albumin levels, tumor burden, region or race, mild or moderate renal impairment [estimated glomerular filtration rate (eGFR) 30 to 89 mL/min/1.73 m2], mild hepatic impairment (bilirubin ≤ ULN and AST > ULN or bilirubin > 1 to 1.5 × ULN and any AST), moderate hepatic impairment (bilirubin >1.5 to 3× ULN and any AST), level of PD-L1 expression, or performance status.
Pediatric Patients
Atezolizumab serum concentrations with weight-based dosing at 15 mg/kg up to a maximum of 1200 mg every 3 weeks, in pediatric patients (2 years to <17 years) with relapsed or progressive solid tumors and lymphomas, are comparable to those of adult patients receiving 1200 mg every 3 weeks; while the exposure tended to be lower in pediatric patients less than 12 years old, this is not considered to be clinically relevant.
12.6 Immunogenicity
The observed incidence of anti-drug antibodies (ADA) is highly dependent on the sensitivity and specificity of the assay. Differences in assay methods preclude meaningful comparisons of the incidence of ADA in the studies described below with the incidence of ADA in other products.
During the first year of treatment with TECENTRIQ across 8 clinical studies, 13% to 36% of patients developed anti-atezolizumab antibodies. Median atezolizumab clearance in patients who tested positive for ADA was 19% (minimum 18%, maximum 49%) higher as compared to atezolizumab clearance in patients who tested negative for ADA; this change in clearance is not expected to be clinically significant.
In OAK and IMbrave150, exploratory analyses showed that the subset of patients who were ADA-positive appeared to have less efficacy (effect on overall survival) as compared to patients who tested negative for ADA [see Clinical Studies (14.1, 14.3)]. In study IMpower150, the impact of ADA on efficacy did not appear to be clinically significant [see Clinical Studies (14.1)]. In the remaining studies, there is insufficient information to characterize the effect of ADA on efficacy.
The presence of ADA did not have a clinically significant effect on the incidence or severity of adverse reactions.
Across clinical studies, 4.3% to 27.5% of neutralizing antibody (NAb)-evaluable patients had a positive NAb status at any timepoint post-treatment. The effect of NAb on atezolizumab exposure and safety did not appear to be clinically significant. The effect of NAb on key efficacy endpoints is uncertain due to small sample sizes.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
No studies have been performed to test the potential of atezolizumab for carcinogenicity or genotoxicity.
Animal fertility studies have not been conducted with atezolizumab; however, an assessment of the male and female reproductive organs was included in a 26-week, repeat-dose toxicity study in cynomolgus monkeys. Weekly administration of atezolizumab to female monkeys at the highest dose tested caused an irregular menstrual cycle pattern and a lack of newly formed corpora lutea in the ovaries. This effect occurred at an estimated AUC approximately 6 times the AUC in patients receiving the recommended dose and was reversible. There was no effect on the male monkey reproductive organs.
13.2 Animal Toxicology and/or Pharmacology
In animal models, inhibition of PD-L1/PD-1 signaling increased the severity of some infections and enhanced inflammatory responses. M. tuberculosis-infected PD-1 knockout mice exhibit markedly decreased survival compared with wild-type controls, which correlated with increased bacterial proliferation and inflammatory responses in these animals. PD-L1 and PD-1 knockout mice and mice receiving PD-L1 blocking antibody have also shown decreased survival following infection with lymphocytic choriomeningitis virus.
-
14 CLINICAL STUDIES
14.1 Non-Small Cell Lung Cancer
Adjuvant Treatment of Stage II-IIIA NSCLC with PD-L1 Expression ≥ 1%
The efficacy of TECENTRIQ was evaluated in IMpower010 (NCT02486718), a multi-center, randomized, open-label trial for the adjuvant treatment of patients with NSCLC who had complete tumor resection and were eligible to receive cisplatin-based adjuvant chemotherapy. Eligible patients were required to have Stage IB (tumors ≥ 4 cm) – Stage IIIA NSCLC per the Union for International Cancer Control/American Joint Committee on Cancer staging system, 7th edition. Patients were excluded if they had a history of autoimmune disease; a history of idiopathic pulmonary fibrosis, organizing pneumonia, drug-induced pneumonitis, idiopathic pneumonitis, or evidence of active pneumonitis; administration of a live, attenuated vaccine within 28 days prior to randomization; administration of systemic immunostimulatory agents within 4 weeks or systemic immunosuppressive medications within 2 weeks prior to randomization.
A total of 1005 patients who had complete tumor resection and received cisplatin-based adjuvant chemotherapy were randomized (1:1) to receive TECENTRIQ 1200 mg intravenous infusion every 3 weeks for 16 cycles, unless disease recurrence or unacceptable toxicity occurred, or best supportive care (BSC). Randomization was stratified by sex, stage of disease, histology, and PD-L1 expression.
Tumor assessments were conducted at baseline of the randomization phase and every 4 months for the first year following Cycle 1, Day 1 and then every 6 months until year five, then annually thereafter.
The median age was 62 years (range: 26 to 84), and 67% of patients were male. The majority of patients were White (73%) and Asian (24%). Most patients were current or previous smokers (78%) and baseline ECOG performance status in patients was 0 (55%) or 1 (44%). Overall, 12% of patients had Stage IB, 47% had Stage II and 41% had Stage IIIA disease. PD-L1 expression, defined as the percentage of tumor cells expressing PD-L1 as measured by the VENTANA PD-L1 (SP263) assay, was ≥ 1% in 53% of patients, <1% in 44% and unknown in 2.6%.
The primary efficacy outcome measure was disease-free survival (DFS) as assessed by the investigator. The primary efficacy analysis population (n = 476) was patients with Stage II – IIIA NSCLC with PD-L1 expression on ≥ 1% of tumor cells (PD-L1 ≥ 1% TC). DFS was defined as the time from the date of randomization to the date of occurrence of any of the following: first documented recurrence of disease, new primary NSCLC, or death due to any cause, whichever occurred first. A key secondary efficacy outcome measure was overall survival (OS) in the intent-to-treat population.
At the time of the interim DFS analysis, the study demonstrated a statistically significant improvement in DFS in the PD-L1 ≥ 1% TC, Stage II – IIIA patient population.
Efficacy results are presented in Table 22 and Figure 1.
Table 22 Efficacy Results from IMpower010 in Patients with Stage II - IIIA NSCLC with PD-L1 expression ≥ 1% TC Arm A:
TECENTRIQ
N = 248Arm B:
Best Supportive Care
N = 228CI = Confidence interval, NE = Not estimable, NR = Not reached - *
- Stratified by stage, sex, and histology
Disease-Free Survival Number of events (%) 88 (35) 105 (46) Median, months NR 35.3 (95% CI) (36.1, NE) (29.0, NE) Hazard ratio* (95% CI) 0.66 (0.50, 0.88) p-value 0.004 In a pre-specified secondary subgroup analysis of patients with PD-L1 TC ≥ 50% Stage II – IIIA NSCLC (n=229), the median DFS was not reached (95% CI: 42.3 months, NE) for patients in the TECENTRIQ arm and was 35.7 months (95% CI: 29.7, NE) for patients in the best supportive care arm, with a HR of 0.43 (95% CI: 0.27, 0.68). In an exploratory subgroup analysis of patients with PD-L1 TC 1-49% Stage II – IIIA NSCLC (n=247), the median DFS was 32.8 months (95% CI: 29.4, NE) for patients in the TECENTRIQ arm and 31.4 months (95% CI: 24.0, NE) for patients in the best supportive care arm, with a HR of 0.87 (95% CI: 0.60, 1.26).
Figure 1: Kaplan-Meier Plot of Disease-Free Survival in IMpower010 in Patients with Stage II – IIIA NSCLC with PD-L1 expression ≥ 1% TC
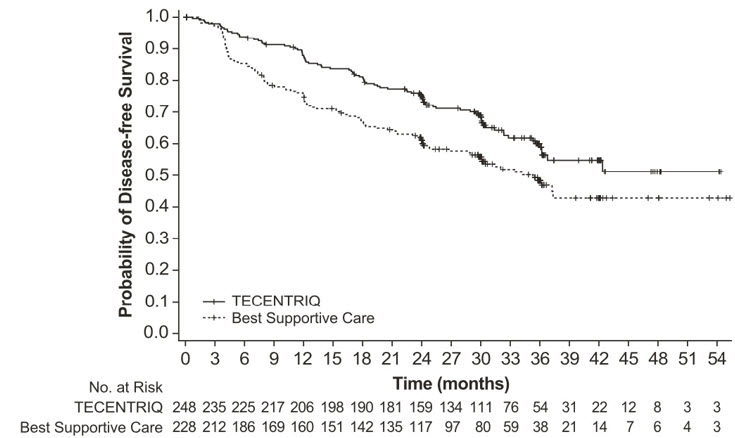
At the time of the DFS interim analysis, 19% of patients in the PD-L1 ≥1% TC Stage II – IIIA patient population had died. An exploratory analysis of OS in this population resulted in a stratified HR of 0.77 (95% CI: 0.51, 1.17).
Metastatic Chemotherapy-Naïve NSCLC with High PD-L1 Expression
The efficacy of TECENTRIQ was evaluated in IMpower110 (NCT02409342), a multicenter, international, randomized, open-label trial in patients with stage IV NSCLC whose tumors express PD-L1 (PD-L1 stained ≥ 1% of tumor cells [TC ≥ 1%] or PD-L1 stained tumor-infiltrating immune cells [IC] covering ≥ 1% of the tumor area [IC ≥ 1%]), who had received no prior chemotherapy for metastatic disease. PD-L1 tumor status was determined based on immunohistochemistry (IHC) testing using the VENTANA PD-L1 (SP142) Assay. The evaluation of efficacy is based on the subgroup of patients with high PD-L1 expression (TC ≥ 50% or IC ≥ 10%), excluding those with EGFR or ALK genomic tumor aberrations. The trial excluded patients with a history of autoimmune disease, administration of a live attenuated vaccine within 28 days prior to randomization, active or untreated CNS metastases, administration of systemic immunostimulatory agents within 4 weeks or systemic immunosuppressive medications within 2 weeks prior to randomization.
Randomization was stratified by sex, ECOG performance status, histology (non-squamous vs. squamous) and PD-L1 expression (TC ≥ 1% and any IC vs. TC < 1% and IC ≥ 1%). Patients were randomized (1:1) to receive one of the following treatment arms:
- Arm A: TECENTRIQ 1200 mg every 3 weeks until disease progression or unacceptable toxicity
- Arm B: Platinum-based chemotherapy
Arm B platinum-based chemotherapy regimens for non-squamous NSCLC consisted of cisplatin (75 mg/m2) and pemetrexed (500 mg/m2) OR carboplatin (AUC 6 mg/mL/min) and pemetrexed (500 mg/m2) on Day 1 of each 21-day cycle for a maximum of 4 or 6 cycles followed by pemetrexed (500 mg/m2) until disease progression or unacceptable toxicity.
Arm B platinum-based chemotherapy regimens for squamous NSCLC consisted of cisplatin (75 mg/m2) on Day 1 with gemcitabine (1250 mg/m2) on Days 1 and 8 of each 21-day cycle OR carboplatin (AUC 5 mg/mL/min) on Day 1 with gemcitabine (1000 mg/m2) on Days 1 and 8 of each 21-day cycle for a maximum of 4 or 6 cycles followed by best supportive care until disease progression or unacceptable toxicity.
Administration of TECENTRIQ was permitted beyond RECIST-defined disease progression. Tumor assessments were conducted every 6 weeks for the first 48 weeks following Cycle 1, Day 1 and then every 9 weeks thereafter. Tumor specimens were evaluated prospectively using the VENTANA PD-L1 (SP142) Assay at a central laboratory and the results were used to define subgroups for pre-specified analyses.
The major efficacy outcome measure was overall survival (OS) sequentially tested in the following subgroups of patients, excluding those with EGFR or ALK genomic tumor aberrations: TC ≥50% or IC ≥10%; TC ≥5% or IC ≥5%; and TC ≥1% or IC ≥1%.
Among the 205 chemotherapy-naïve patients with stage IV NSCLC with high PD-L1 expression (TC ≥ 50% or IC ≥ 10%) excluding those with EGFR or ALK genomic tumor aberrations, the median age was 65.0 years (range: 33 to 87), and 70% of patients were male. The majority of patients were White (82%) and Asian (17%). Baseline ECOG performance status was 0 (36%) or 1 (64%); 88% were current or previous smokers; and 76% of patients had non-squamous disease while 24% of patients had squamous disease.
The trial demonstrated a statistically significant improvement in OS for patients with high PD-L1 expression (TC ≥50% or IC ≥10%) at the time of the OS interim analysis. There was no statistically significant difference in OS for the other two PD-L1 subgroups (TC ≥5% or IC ≥5%; and TC ≥1% or IC ≥1%) at the interim or final analyses. Efficacy results for patients with NSCLC with high PD-L1 expression are presented in Table 23 and Figure 2.
Table 23: Efficacy Results from IMpower110 in Patients with NSCLC with High PD-L1 Expression (TC ≥ 50% or IC ≥ 10%) and without EGFR or ALK Genomic Tumor Aberrations Arm A: TECENTRIQ
N = 107Arm B: Platinum-Based Chemotherapy
N = 98CI=confidence interval; NE=not estimable Overall Survival* Deaths (%) 44 (41%) 57 (58%) Median, months 20.2 13.1 (95% CI) (16.5, NE) (7.4, 16.5) Hazard ratio† (95% CI) 0.59 (0.40, 0.89) p-value‡ 0.0106§ Figure 2: Kaplan-Meier Plot of Overall Survival in IMpower110 in Patients with NSCLC with High PD-L1 Expression (TC ≥ 50% or IC ≥ 10%) and without EGFR or ALK Genomic Tumor Aberrations
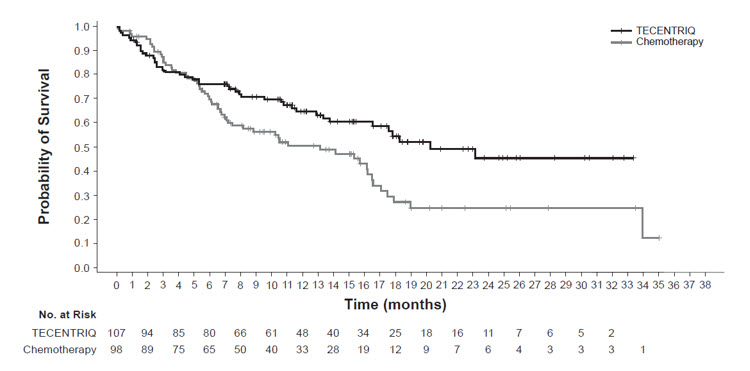
Investigator-assessed PFS showed an HR of 0.63 (95% CI: 0.45, 0.88), with median PFS of 8.1 months (95% CI: 6.8, 11.0) in the TECENTRIQ arm and 5 months (95% CI: 4.2, 5.7) in the platinum-based chemotherapy arm. The investigator-assessed confirmed ORR was 38% (95% CI: 29%, 48%) in the TECENTRIQ arm and 29% (95% CI: 20%, 39%) in the platinum-based chemotherapy arm.
Metastatic Chemotherapy-Naive Non-Squamous NSCLC
IMpower150
The efficacy of TECENTRIQ with bevacizumab, paclitaxel, and carboplatin was evaluated in IMpower150 (NCT02366143), a multicenter, international, randomized (1:1:1), open-label trial in patients with metastatic non-squamous NSCLC. Patients with stage IV non-squamous NSCLC who had received no prior chemotherapy for metastatic disease but could have received prior EGFR or ALK kinase inhibitor if appropriate, regardless of PD-L1 or T-effector gene (tGE) status and ECOG performance status 0 or 1 were eligible. The trial excluded patients with a history of autoimmune disease, administration of a live attenuated vaccine within 28 days prior to randomization, active or untreated CNS metastases, administration of systemic immunostimulatory agents within 4 weeks or systemic immunosuppressive medications within 2 weeks prior to randomization, or clear tumor infiltration into the thoracic great vessels or clear cavitation of pulmonary lesions as seen on imaging. Randomization was stratified by sex, presence of liver metastases, and PD-L1 expression status on tumor cells (TC) and tumor-infiltrating immune cells (IC) as follows: TC3 and any IC vs. TC0/1/2 and IC2/3 vs. TC0/1/2 and IC0/1. Patients were randomized to one of the following three treatment arms:
- Arm A: TECENTRIQ 1200 mg, paclitaxel 175 mg/m2 or 200 mg/m2 and carboplatin AUC 6 mg/mL/min on Day 1 of each 21-day cycle for a maximum of 4 or 6 cycles
- Arm B: TECENTRIQ 1200 mg, bevacizumab 15 mg/kg, paclitaxel 175 mg/m2 or 200 mg/m2, and carboplatin AUC 6 mg/mL/min on Day 1 of each 21-day cycle for a maximum of 4 or 6 cycles
- Arm C: bevacizumab 15 mg/kg, paclitaxel 175 mg/m2 or 200 mg/m2, and carboplatin AUC 6 mg/mL/min on Day 1 of each 21-day cycle for a maximum of 4 or 6 cycles
Patients who had not experienced disease progression following the completion or cessation of platinum-based chemotherapy, received:
- Arm A: TECENTRIQ 1200 mg intravenously on Day 1 of each 21-day cycle until disease progression or unacceptable toxicity
- Arm B: TECENTRIQ 1200 mg and bevacizumab 15 mg/kg intravenously on Day 1 of each 21-day cycle until disease progression or unacceptable toxicity
- Arm C: bevacizumab 15 mg/kg intravenously on Day 1 of each 21-day cycle until disease progression or unacceptable toxicity
Tumor assessments were conducted every 6 weeks for the first 48 weeks following Cycle 1, Day 1 and then every 9 weeks thereafter. Tumor specimens were evaluated prior to randomization for PD-L1 tumor expression using the VENTANA PD-L1 (SP142) assay at a central laboratory. Tumor tissue was collected at baseline for expression of tGE signature and evaluation was performed using a clinical trial assay in a central laboratory prior to the analysis of efficacy outcome measures.
Major efficacy outcome measures for comparison of Arms B and C were progression free survival (PFS) by RECIST v1.1 in the tGE-WT (patients with high expression of T-effector gene signature [tGE], excluding those with EGFR- and ALK-positive NSCLC [WT]) and in the ITT-WT subpopulations and overall survival (OS) in the ITT-WT subpopulation. Additional efficacy outcome measures for comparison of Arms B and C or Arms A and C were PFS and OS in the ITT population, OS in the tGE-WT subpopulation, and ORR/DoR in the tGE-WT and ITT-WT subpopulations.
A total of 1202 patients were enrolled across the three arms of whom 1045 were in the ITT-WT subpopulation and 447 were in the tGE-WT subpopulation. The demographic information is limited to the 800 patients enrolled in Arms B and C where efficacy has been demonstrated. The median age was 63 years (range: 31 to 90), and 60% of patients were male. The majority of patients were White (82%), 13% of patients were Asian, 10% were Hispanic, and 2% of patients were Black. Clinical sites in Asia (enrolling 13% of the study population) received paclitaxel at a dose of 175 mg/m2 while the remaining 87% received paclitaxel at a dose of 200 mg/m2. Approximately 14% of patients had liver metastases at baseline, and most patients were current or previous smokers (80%). Baseline ECOG performance status was 0 (43%) or 1 (57%). PD-L1 was TC3 and any IC in 12%, TC0/1/2 and IC2/3 in 13%, and TC0/1/2 and IC0/1 in 75%. The demographics for the 696 patients in the ITT-WT subpopulation were similar to the ITT population except for the absence of patients with EGFR- or ALK- positive NSCLC.
The trial demonstrated a statistically significant improvement in PFS between Arms B and C in both the tGE-WT and ITT-WT subpopulations, but did not demonstrate a significant difference for either subpopulation between Arms A and C based on the final PFS analyses. In the interim analysis of OS, a statistically significant improvement was observed for Arm B compared to Arm C, but not for Arm A compared to Arm C. Efficacy results for the ITT-WT subpopulation are presented in Table 24 and Figure 3.
Table 24: Efficacy Results in ITT-WT Population in IMpower150 Arm C: Bevacizumab, Paclitaxel and Carboplatin Arm B: TECENTRIQ with Bevacizumab, Paclitaxel, and Carboplatin Arm A: TECENTRIQ with Paclitaxel, and Carboplatin N = 337 N = 359 N = 349 CI=confidence interval - *
- Based on OS interim analysis
- †
- Stratified by sex, presence of liver metastases, and PD-L1 expression status on TC and IC
- ‡
- Based on the stratified log-rank test compared to Arm C
- §
- Compared to the allocated α=0.0174 (two sided) for this interim analysis
- ¶
- Compared to the allocated α=0.0128 (two sided) for this interim analysis
- #
- As determined by independent review facility (IRF) per RECIST v1.1 (Response Evaluation Criteria in Solid Tumors v1.1)
- Þ
- Compared to the allocated α=0.006 (two sided) for the final PFS analysis
Overall Survival* Deaths (%) 197 (59%) 179 (50%) 179 (51%) Median, months 14.7 19.2 19.4 (95% CI) (13.3, 16.9) (17.0, 23.8) (15.7, 21.3) Hazard ratio† (95% CI) --- 0.78 (0.64, 0.96) 0.84 (0.72, 1.08) p-value‡ --- 0.016§ 0.204¶ Progression-Free Survival# Number of events (%) 247 (73%) 247 (69%) 245 (70%) Median, months 7.0 8.5 6.7 (95% CI) (6.3, 7.9) (7.3, 9.7) (5.6, 6.9) Hazard ratio† (95% CI) --- 0.71 (0.59, 0.85) 0.94 (0.79, 1.13) p-value‡ --- 0.0002Þ 0.5219 Objective Response Rate# Number of responders (%) 142 (42%) 196 (55%) 150 (43%) (95% CI) (37, 48) (49, 60) (38, 48) Complete Response 3 (1%) 14 (4%) 9 (3%) Partial Response 139 (41%) 182 (51%) 141 (40%) Duration of Response# n = 142 n = 196 n = 150 Median, months 6.5 10.8 9.5 (95% CI) (5.6, 7.6) (8.4, 13.9) (7.0, 13.0) Figure 3: Kaplan-Meier Curves for Overall Survival in ITT-WT Population in IMpower150
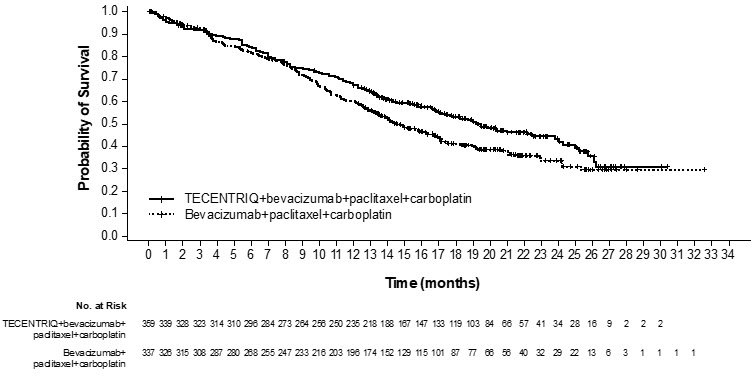
Exploratory analyses showed that the subset of patients in the four drug regimen arm who were ADA positive by week 4 (30%) appeared to have similar efficacy (effect on overall survival) as compared to patients who tested negative for treatment-emergent ADA by week 4 (70%) [see, Clinical Pharmacology (12.6)]. In an exploratory analysis, propensity score matching was conducted to compare ADA positive patients in the TECENTRIQ, bevacizumab, paclitaxel, and carboplatin arm with a matched population in the bevacizumab, paclitaxel, and carboplatin arm. Similarly ADA negative patients in the TECENTRIQ, bevacizumab, paclitaxel, and carboplatin arm were compared with a matched population in the bevacizumab, paclitaxel, and carboplatin arm. Propensity score matching factors were: baseline sum of longest tumor size (BSLD), baseline ECOG, baseline albumin, baseline LDH, sex, tobacco history, metastatic site, TC level, and IC level. The hazard ratio comparing the ADA-positive subgroup with its matched control was 0.69 (95% CI: 0.44, 1.07). The hazard ratio comparing the ADA-negative subgroup with its matched control was 0.64 (95% CI: 0.46, 0.90).
IMpower130
The efficacy of TECENTRIQ with paclitaxel protein-bound and carboplatin was evaluated in IMpower130 (NCT02367781), a multicenter, randomized (2:1), open-label trial in patients with stage IV non-squamous NSCLC. Patients with Stage IV non-squamous NSCLC who had received no prior chemotherapy for metastatic disease, but could have received prior EGFR or ALK kinase inhibitor, if appropriate, were eligible. The trial excluded patients with history of autoimmune disease, administration of live attenuated vaccine within 28 days prior to randomization, administration of immunostimulatory agents within 4 weeks or systemic immunosuppressive medications within 2 weeks prior to randomization, and active or untreated CNS metastases. Randomization was stratified by sex, presence of liver metastases, and PD-L1 tumor expression according to the VENTANA PD-L1 (SP142) assay as follows: TC3 and any IC vs. TC0/1/2 and IC2/3 vs. TC0/1/2 and IC0/1. Patients were randomized to one of the following treatment regimens:
- TECENTRIQ 1200 mg on Day 1, paclitaxel protein-bound 100 mg/m2 on Days 1, 8, and 15, and carboplatin AUC 6 mg/mL/min on Day 1 of each 21-day cycle for a maximum of 4 or 6 cycles followed by TECENTRIQ 1200 mg once every 3 weeks until disease progression or unacceptable toxicity, or
- Paclitaxel protein-bound 100 mg/m2 on Days 1, 8 and 15 and carboplatin AUC 6 mg/mL/min on Day 1 of each 21-day cycle for a maximum of 4 or 6 cycles followed by best supportive care or pemetrexed.
Tumor assessments were conducted every 6 weeks for the first 48 weeks, then every 9 weeks thereafter. Major efficacy outcome measures were PFS by RECIST v1.1 and OS in the subpopulation of patients evaluated for and documented to have no EGFR or ALK genomic tumor aberrations (ITT-WT).
A total of 724 patients were enrolled; of these, 681 (94%) were in the ITT-WT population. The median age was 64 years (range: 18 to 86) and 59% were male. The majority of patients were white (90%), 2% of patients were Asian, 5% were Hispanic, and 4% were Black. Baseline ECOG performance status was 0 (41%) or 1 (58%). Most patients were current or previous smokers (90%). PD-L1 tumor expression was TC0/1/2 and IC0/1 in 73%; TC3 and any IC in 14%; and TC0/1/2 and IC2/3 in 13%.
Efficacy results for the ITT-WT population are presented in Table 25 and Figure 4.
Table 25: Efficacy Results from IMpower130 TECENTRIQ with Paclitaxel Protein-Bound and Carboplatin Paclitaxel Protein-Bound and Carboplatin CI=confidence interval - *
- Based on OS interim analysis
- †
- Stratified by sex and PD-L1 tumor expression on tumor cells (TC) and tumor infiltrating cells (IC)
- ‡
- Based on the stratified log-rank test
- §
- Compared to the allocated α=0.0428 (two sided) for this interim analysis
- ¶
- As determined by independent review facility (IRF) per RECIST v1.1 (Response Evaluation Criteria in Solid Tumors v1.1)
- #
- Compared to the allocated α=0.006 (two sided) for the final PFS analysis
- Þ
- Confirmed response
Overall Survival* n=453 n=228 Deaths (%) 228 (50%) 131 (57%) Median, months 18.6 13.9 (95% CI) (15.7, 21.1) (12.0, 18.7) Hazard ratio† (95% CI) 0.80 (0.64, 0.99) p-value‡ 0.0384§ Progression-Free Survival¶ n=453 n=228 Number of events (%) 330 (73%) 177 (78%) Median, months 7.2 6.5 (95% CI) (6.7, 8.3) (5.6, 7.4) Hazard ratio† (95% CI) 0.75 (0.63, 0.91) p-value‡ 0.0024# Overall Response Rate¶,Þ n=453 n=228 Number of responders (%) 207 (46%) 74 (32%) (95% CI) (41, 50) (26, 39) Complete Response 22 (5%) 2 (1%) Partial Response 185 (41%) 72 (32%) Duration of Response¶,Þ n=207 n=74 Median, months 10.8 7.8 (95% CI) (9.0, 14.4) (6.8, 10.9) Figure 4: Kaplan-Meier Curves for Overall Survival in IMpower130
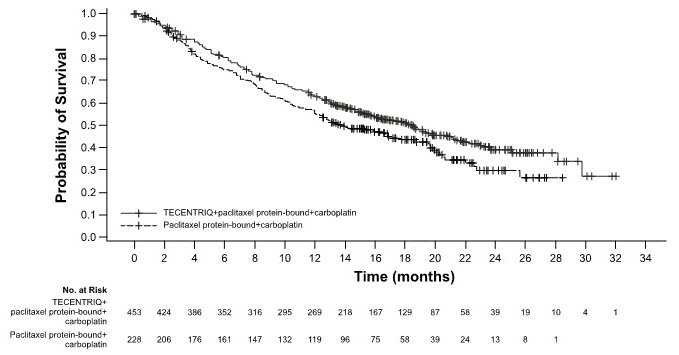
Previously Treated Metastatic NSCLC
The efficacy of TECENTRIQ was evaluated in a multicenter, international, randomized (1:1), open-label study (OAK; NCT02008227) conducted in patients with locally advanced or metastatic NSCLC whose disease progressed during or following a platinum-containing regimen. Patients with a history of autoimmune disease, symptomatic or corticosteroid-dependent brain metastases, or requiring systemic immunosuppression within 2 weeks prior to enrollment were ineligible. Randomization was stratified by PD-L1 expression tumor-infiltrating immune cells (IC), the number of prior chemotherapy regimens (1 vs. 2), and histology (squamous vs. non-squamous).
Patients were randomized to receive TECENTRIQ 1200 mg intravenously every 3 weeks until unacceptable toxicity, radiographic progression, or clinical progression or docetaxel 75 mg/m2 intravenously every 3 weeks until unacceptable toxicity or disease progression. Tumor assessments were conducted every 6 weeks for the first 36 weeks and every 9 weeks thereafter. Major efficacy outcome measure was overall survival (OS) in the first 850 randomized patients and OS in the subgroup of patients with PD-L1-expressing tumors (defined as ≥ 1% PD-L1 expression on tumor cells [TC] or immune cells [IC]). Additional efficacy outcome measures were OS in all randomized patients (n = 1225), OS in subgroups based on PD-L1 expression, overall response rate (ORR), and progression free survival as assessed by the investigator per RECIST v.1.1.
Among the first 850 randomized patients, the median age was 64 years (33 to 85 years) and 47% were ≥ 65 years old; 61% were male; 70% were White and 21% were Asian; 15% were current smokers and 67% were former smokers; and 37% had baseline ECOG PS of 0 and 63% had a baseline ECOG PS of 1. Nearly all (94%) had metastatic disease, 74% had non-squamous histology, 75% had received only one prior platinum-based chemotherapy regimen, and 55% of patients had PD-L1-expressing tumors.
Efficacy results are presented in Table 26 and Figure 5.
Table 26: Efficacy Results in OAK TECENTRIQ Docetaxel CI=confidence interval; NE=not estimable - *
- Stratified by PD-L1 expression in tumor infiltrating immune cells, the number of prior chemotherapy regimens, and histology
- †
- Based on the stratified log-rank test
- ‡
- Compared to the pre-specified allocated α of 0.03 for this analysis
- §
- Per RECIST v1.1 (Response Evaluation Criteria in Solid Tumors v1.1)
- ¶
- Compared to the allocated α of 0.0177 for this interim analysis based on 86% information using O'Brien-Fleming boundary
Overall Survival in first 850 patients Number of patients N=425 N=425 Deaths (%) 271 (64%) 298 (70%) Median, months 13.8 9.6 (95% CI) (11.8, 15.7) (8.6, 11.2) Hazard ratio* (95% CI) 0.74 (0.63, 0.87) p-value† 0.0004‡ Progression-Free Survival Number of Patients N=425 N=425 Events (%) 380 (89%) 375 (88%) Progression (%) 332 (78%) 290 (68%) Deaths (%) 48 (11%) 85 (20%) Median, months 2.8 4.0 (95% CI) (2.6, 3.0) (3.3, 4.2) Hazard ratio* (95% CI) 0.95 (0.82, 1.10) Overall Response Rate§ Number of Patients N=425 N=425 ORR, n (%) 58 (14%) 57 (13%) (95% CI) (11%, 17%) (10%, 17%) Complete Response 6 (1%) 1 (0.2%) Partial Response 52 (12%) 56 (13%) Duration of Response‡ N=58 N=57 Median, months 16.3 6.2 (95% CI) (10.0, NE) (4.9, 7.6) Overall Survival in all 1225 patients Number of patients N=613 N=612 Deaths (%) 384 (63%) 409 (67%) Median, months 13.3 9.8 (95% CI) (11.3, 14.9) (8.9, 11.3) Hazard ratio* (95% CI) 0.79 (0.69, 0.91) p-value† 0.0013¶ Figure 5: Kaplan-Meier Curves of Overall Survival in the First 850 Patients Randomized in OAK 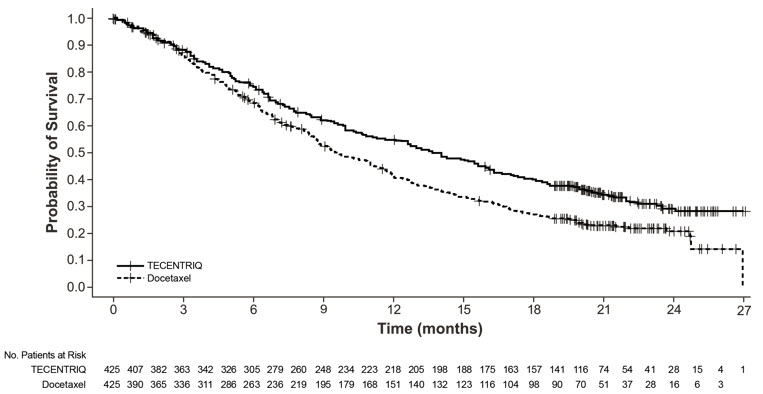
Tumor specimens were evaluated prospectively using the VENTANA PD-L1 (SP142) Assay at a central laboratory and the results were used to define the PD-L1 expression subgroups for pre-specified analyses. Of the 850 patients, 16% were classified as having high PD-L1 expression, defined as having PD-L1 expression on ≥ 50% of TC or ≥ 10% of IC. In an exploratory efficacy subgroup analysis of OS based on PD-L1 expression, the hazard ratio was 0.41 (95% CI: 0.27, 0.64) in the high PD-L1 expression subgroup and 0.82 (95% CI: 0.68, 0.98) in patients who did not have high PD-L1 expression.
Exploratory analyses showed that the subset of patients who were ADA positive by week 4 (21%) appeared to have less efficacy (effect on overall survival) as compared to patients who tested negative for treatment-emergent ADA by week 4 (79%) [see Clinical Pharmacology (12.6)]. ADA positive patients by week 4 appeared to have similar OS compared to docetaxel-treated patients. In an exploratory analysis, propensity score matching was conducted to compare ADA positive patients in the atezolizumab arm with a matched population in the docetaxel arm and ADA negative patients in the atezolizumab arm with a matched population in the docetaxel arm. Propensity score matching factors were: baseline sum of longest tumor size (BSLD), baseline ECOG, histology (squamous vs. non-squamous), baseline albumin, baseline LDH, gender, tobacco history, metastases status (advanced or local), metastatic site, TC level, and IC level. The hazard ratio comparing the ADA positive subgroup with its matched control was 0.89 (95% CI: 0.61, 1.3). The hazard ratio comparing the ADA negative subgroup with its matched control was 0.68 (95% CI: 0.55, 0.83).
14.2 Small Cell Lung Cancer
The efficacy of TECENTRIQ with carboplatin and etoposide was investigated in IMpower133 (NCT02763579), a randomized (1:1), multicenter, double-blind, placebo-controlled trial in 403 patients with ES-SCLC. IMpower133 enrolled patients with ES-SCLC who had received no prior chemotherapy for extensive stage disease and ECOG performance status 0 or 1. The trial excluded patients with active or untreated CNS metastases, history of autoimmune disease, administration of a live, attenuated vaccine within 4 weeks prior to randomization, or administration of systemic immunosuppressive medications within 1 week prior to randomization. Randomization was stratified by sex, ECOG performance status, and presence of brain metastases. Patients were randomized to receive one of the following two treatment arms:
- TECENTRIQ 1200 mg and carboplatin AUC 5 mg/mL/min on Day 1 and etoposide 100 mg/m2 intravenously on Days 1, 2 and 3 of each 21-day cycle for a maximum of 4 cycles followed by TECENTRIQ 1200 mg once every 3 weeks until disease progression or unacceptable toxicity, or
- placebo and carboplatin AUC 5 mg/mL/min on Day 1 and etoposide 100 mg/m2 intravenously on Days 1, 2, and 3 of each 21-day cycle for a maximum of 4 cycles followed by placebo once every 3 weeks until disease progression or unacceptable toxicity.
Administration of TECENTRIQ was permitted beyond RECIST-defined disease progression. Tumor assessments were conducted every 6 weeks for the first 48 weeks following Cycle 1, Day 1 and then every 9 weeks thereafter. Patients treated beyond disease progression had tumor assessment conducted every 6 weeks until treatment discontinuation.
Major efficacy outcome measures were OS and PFS as assessed by investigator per RECIST v1.1 in the intent-to-treat population. Additional efficacy outcome measures included ORR and DoR as assessed by investigator per RECIST v1.1.
A total of 403 patients were randomized, including 201 to the TECENTRIQ arm and 202 to the chemotherapy alone arm. The median age was 64 years (range 26 to 90) and 65% were male. The majority of patients were White (80%); 17% were Asian, 4% were Hispanic and 1% were Black. Baseline ECOG performance status was 0 (35%) or 1 (65%); 9% of patients had a history of brain metastases, and 97% were current or previous smokers.
Efficacy results are presented in Table 27 and Figure 6.
Table 27: Efficacy Results from IMpower133 TECENTRIQ with Carboplatin and Etoposide Placebo with Carboplatin and Etoposide CI=confidence interval - *
- Stratified by sex and ECOG performance status
- †
- Based on the stratified log-rank test
- ‡
- Compared to the allocated α of 0.0193 for this interim analysis based on 78% information using O'Brien-Fleming boundary
- §
- As determined by investigator assessment
- ¶
- per RECIST v1.1 (Response Evaluation Criteria in Solid Tumors v1.1)
- #
- Compared to the allocated α of 0.05 for this analysis
- Þ
- Confirmed response
Overall Survival N=201 N=202 Deaths (%) 104 (52%) 134 (66%) Median, months 12.3 10.3 (95% CI) (10.8, 15.9) (9.3, 11.3) Hazard ratio* (95% CI) 0.70 (0.54, 0.91) p-value†, ‡ 0.0069 Progression-Free Survival§,¶ N=201 N=202 Number of events (%) 171 (85%) 189 (94%) Median, months 5.2 4.3 (95% CI) (4.4, 5.6) (4.2, 4.5) Hazard ratio* (95% CI) 0.77 (0.62, 0.96) p-value†, # 0.0170 Objective Response Rate§,¶,Þ N=201 N=202 Number of responders (%) 121 (60%) 130 (64%) (95% CI) (53, 67) (57, 71) Complete Response (%) 5 (2%) 2 (1%) Partial Response (%) 116 (58%) 128 (63%) Duration of Response§,¶,Þ N=121 N=130 Median, months 4.2 3.9 (95% CI) (4.1, 4.5) (3.1, 4.2) Figure 6: Kaplan-Meier Plot of Overall Survival in IMpower133
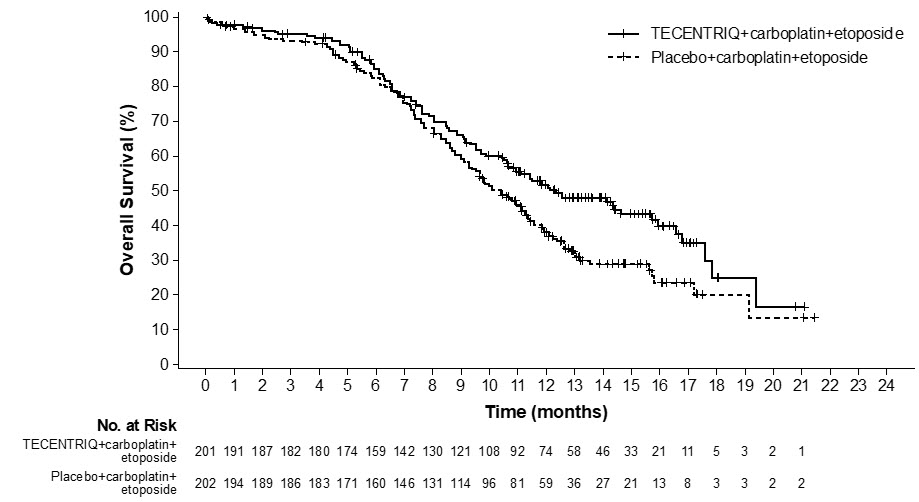
14.3 Hepatocellular Carcinoma
The efficacy of TECENTRIQ in combination with bevacizumab was investigated in IMbrave150 (NCT03434379), a multicenter, international, open-label, randomized trial in patients with locally advanced unresectable and/or metastatic hepatocellular carcinoma who have not received prior systemic therapy. Randomization was stratified by geographic region (Asia excluding Japan vs. rest of world), macrovascular invasion and/or extrahepatic spread (presence vs. absence), baseline AFP (<400 vs. ≥400 ng/mL), and by ECOG performance status (0 vs. 1).
A total of 501 patients were randomized (2:1) to receive either TECENTRIQ as an intravenous infusion of 1200 mg, followed by 15 mg/kg bevacizumab, on the same day every 3 weeks or sorafenib 400 mg given orally twice daily, until disease progression or unacceptable toxicity. Patients could discontinue either TECENTRIQ or bevacizumab (e.g., due to adverse events) and continue on single-agent therapy until disease progression or unacceptable toxicity associated with the single-agent.
The study enrolled patients who were ECOG performance score 0 or 1 and who had not received prior systemic treatment. Patients were required to be evaluated for the presence of varices within 6 months prior to treatment, and were excluded if they had variceal bleeding within 6 months prior to treatment, untreated or incompletely treated varices with bleeding, or high risk of bleeding. Patients with Child-Pugh B or C cirrhosis, moderate or severe ascites; history of hepatic encephalopathy; a history of autoimmune disease; administration of a live, attenuated vaccine within 4 weeks prior to randomization; administration of systemic immunostimulatory agents within 4 weeks or systemic immunosuppressive medications within 2 weeks prior to randomization; or untreated or corticosteroid-dependent brain metastases were excluded. Tumor assessments were performed every 6 weeks for the first 54 weeks and every 9 weeks thereafter.
The demographics and baseline disease characteristics of the study population were balanced between the treatment arms. The median age was 65 years (range: 26 to 88) and 83% of patients were male. The majority of patients were Asian (57%) or White (35%); 40% were from Asia (excluding Japan). Approximately 75% of patients presented with macrovascular invasion and/or extrahepatic spread and 37% had a baseline AFP ≥400 ng/mL. Baseline ECOG performance status was 0 (62%) or 1 (38%). HCC risk factors were Hepatitis B in 48% of patients, Hepatitis C in 22%, and 31% of patients had non-viral liver disease. The majority of patients had BCLC stage C (82%) disease at baseline, while 16% had stage B, and 3% had stage A.
The major efficacy outcome measures were overall survival (OS) and independent review facility (IRF)-assessed progression free survival (PFS) per RECIST v1.1. Additional efficacy outcome measures were IRF-assessed overall response rate (ORR) per RECIST and mRECIST.
Efficacy results are presented in Table 28 and Figure 7.
Table 28: Efficacy Results from IMbrave150 TECENTRIQ in combination with Bevacizumab
(N= 336)Sorafenib
(N=165)CI=confidence interval; HCC mRECIST=Modified RECIST Assessment for Hepatocellular Carcinoma; NE=not estimable; RECIST 1.1=Response Evaluation Criteria in Solid Tumors v1.1 - *
- Stratified by geographic region (Asia excluding Japan vs. rest of world), macrovascular invasion and/or extrahepatic spread (presence vs. absence), and baseline AFP (<400 vs. ≥400 ng/mL)
- †
- Based on two-sided stratified log-rank test; as compared to significance level 0.004 (2-sided) based on 161/312=52% information using the OBF method
- ‡
- Per independent radiology review
- §
- Confirmed responses
- ¶
- Based on two-sided Cochran-Mantel-Haesnszel test
- #
- Denotes a censored value
Overall Survival Number of deaths (%) 96 (29) 65 (39) Median OS in months (95% CI) NE
(NE, NE)13.2
(10.4, NE)Hazard ratio* (95% CI) 0.58 (0.42, 0.79) p-value† 0.0006† Progression-Free Survival‡ Number of events (%) 197 (59) 109 (66) Median PFS in months (95% CI) 6.8 (5.8, 8.3) 4.3 (4.0, 5.6) Hazard ratio* (95% CI) 0.59 (0.47, 0.76) p-value <0.0001 Overall Response Rate‡,§(ORR), RECIST 1.1 Number of responders (%) 93 (28) 19 (12) (95% CI) (23, 33) (7,17) p-value¶ <0.0001 Complete responses, n (%) 22 (7) 0 Partial responses, n (%) 71 (21) 19 (12) Duration of Response‡,§ (DOR) RECIST 1.1 (n=93) (n=19) Median DOR in months (95% CI) NE
(NE, NE)6.3
(4.7, NE)Range (months) (1.3#, 13.4#) (1.4#, 9.1#) Overall Response Rate‡,§ (ORR), HCC mRECIST Number of responders (%) 112 (33) 21 (13) (95% CI) (28, 39) (8, 19) p-value¶ <0.0001 Complete responses, n (%) 37 (11) 3 (1.8) Partial responses, n (%) 75 (22) 18 (11) Duration of Response‡,§ (DOR) HCC mRECIST (n=112) (n=21) Median DOR in months
(95% CI)NE
(NE, NE)6.3
(4.9, NE)Range (months) (1.3#, 13.4#) (1.4#, 9.1#) Figure 7: Kaplan-Meier Plot of Overall Survival in IMbrave150
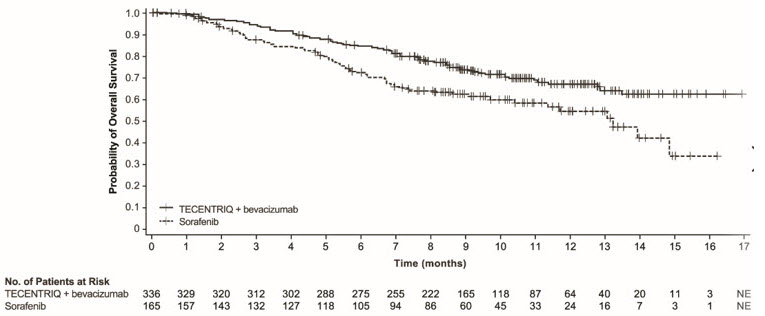
Exploratory analyses showed that the subset of patients (20%) who were ADA-positive by week 6 appeared to have reduced efficacy (effect on OS) as compared to patients (80%) who tested negative for treatment-emergent ADA by week 6 [see Clinical Pharmacology (12.6)]. ADA-positive patients by week 6 appeared to have similar overall survival compared to sorafenib-treated patients. In an exploratory analysis, inverse probability weighting was conducted to compare ADA-positive patients and ADA-negative patients in the TECENTRIQ and bevacizumab arm to the sorafenib arm. Inverse probability weighting factors were: baseline sum of longest tumor size (BSLD), baseline ECOG, baseline albumin, baseline LDH, sex, age, race, geographic region, weight, neutrophil-to-lymphocyte ratio, AFP (<400 ng/mL vs ≥400 ng/mL), number of metastatic sites, MVI and/or EHS present at study entry, etiology (HBV vs. HCV vs. non-viral) and Child-Pugh Score (A5 vs. A6). The OS hazard ratio comparing the ADA-positive subgroup of the TECENTRIQ and bevacizumab arm to sorafenib was 0.93 (95% CI: 0.57, 1.53). The OS hazard ratio comparing the ADA-negative subgroup to sorafenib was 0.39 (95% CI: 0.26, 0.60).
14.4 Melanoma
The efficacy of TECENTRIQ in combination with cobimetinib and vemurafenib was evaluated in a double-blind, randomized (1:1), placebo-controlled, multicenter trial (IMspire150; NCT02908672) conducted in 514 patients. Randomization was stratified by geographic location (North America vs. Europe vs. Australia, New Zealand, and others) and baseline lactate dehydrogenase (LDH) [less than or equal to upper limit of normal (ULN) vs. greater than ULN]. Eligible patients were required to have previously untreated unresectable or metastatic BRAF V600 mutation-positive melanoma as detected by a locally available test and centrally confirmed with the FoundationOne™ assay. Patients were excluded if they had history of autoimmune disease; administration of a live, attenuated vaccine within 28 days prior to randomization; administration of systemic immunostimulatory agents within 4 weeks or systemic immunosuppressive medications within 2 weeks prior to randomization; and active or untreated CNS metastases.
TECENTRIQ was initiated after patients received a 28-day treatment cycle of cobimetinib 60 mg orally once daily (21 days on / 7 days off) and vemurafenib 960 mg orally twice daily Days 1-21 and 720 mg orally twice daily Days 22-28. Patients received TECENTRIQ 840 mg intravenous infusion over 60 minutes every 2 weeks in combination with cobimetinib 60 mg orally once daily and vemurafenib 720 mg orally twice daily, or placebo in combination with cobimetinib 60 mg orally once daily and vemurafenib 960 mg orally twice daily. Treatment continued until disease progression or unacceptable toxicity. There was no crossover at the time of disease progression. Tumor assessments were performed every 8 weeks (± 1 week) for the first 24 months and every 12 weeks (± 1 week) thereafter.
The major efficacy outcome measure was investigator-assessed progression-free survival (PFS) per RECIST v1.1. Additional efficacy outcomes included PFS assessed by an independent central review, investigator-assessed ORR, OS, and DOR.
The median age of the study population was 54 years (range: 22-88), 58% of patients were male, 95% were White, a baseline ECOG performance status of 0 (77%) or 1 (23%), 33% had elevated LDH, 94% had metastatic disease, 60% were Stage IV (M1C), 56% had less than three metastatic sites at baseline, 3% had prior treatment for brain metastases, 30% had liver metastases at baseline, and 14% had received prior adjuvant systemic therapy. Based on central testing, 74% were identified as having a V600E mutation, 11% as having V600K mutation, and 1% as having V600D or V600R mutations.
Efficacy results are summarized in Table 29 and Figure 8. Patients had a median survival follow up time of 18.9 months.
Table 29 Efficacy Results from IMspire150 TECENTRIQ + Cobimetinib + Vemurafenib
N=256Placebo + Cobimetinib + Vemurafenib
N=258Progression-Free Survival* Number of events (%) 148 (58) 179 (69) Median, months 15.1 10.6 (95% CI) (11.4, 18.4) (9.3, 12.7) Hazard ratio† (95% CI) 0.78 (0.63, 0.97) p-value‡ 0.0249 Overall Response Rate*,§ Number of responders (%) 170 (66) 168 (65) (95% CI) (60, 72) (59, 71) Complete responses, n (%) 41 (16) 46 (18) Partial response, n (%) 129 (50) 122 (47) Duration of Response*,§ n=170 n=168 Median, months 20.4 12.5 (95% CI) (15.1, NE) (10.7, 16.6) Figure 8: Kaplan-Meier Plot for Progression-Free Survival in IMspire150
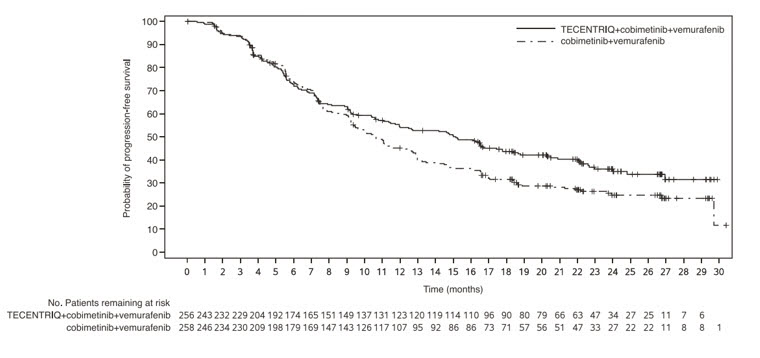
At a pre-specified analysis at the time of the primary analysis of PFS, the OS data were not mature. The median OS was 28.8 months with 93 (36%) deaths in the TECENTRIQ plus cobimetinib and vemurafenib arm, and 25.1 months with 112 (43%) deaths in the placebo plus cobimetinib and vemurafenib arm. The hazard ratio for OS was 0.85 (95% CI: 0.64, 1.11) and the p-value was 0.2310.
14.5 Alveolar soft part sarcoma (ASPS)
The efficacy of TECENTRIQ was evaluated in study ML39345 (NCT03141684), an open-label, single-arm study, in 49 adult and pediatric patients aged 2 years and older with unresectable or metastatic ASPS. Eligible patients were required to have histologically or cytologically confirmed ASPS that was not curable by surgery, and an ECOG performance status of ≤ 2.
Patients were excluded if they had known primary central nervous system (CNS) malignancy or symptomatic CNS metastases, known clinically significant liver disease, or history of idiopathic pulmonary fibrosis, pneumonitis, organizing pneumonia, or evidence of active pneumonitis on screening chest computed tomography (CT) scan.
Adult patients received 1200 mg intravenously and pediatric patients received 15 mg/kg (up to a maximum of 1200 mg) intravenously once every 21 days until disease progression or unacceptable toxicity.
The major efficacy outcomes were Overall Response Rate (ORR) and Duration of Response (DOR) by Independent Review Committee according to Response Evaluation Criteria in Solid Tumors (RECIST) v1.1.
A total of 49 patients were enrolled. The median age of patients was 31 years (range: 12-70); 2% of adult patients (n=47) were ≥65 years of age and the pediatric patients (n=2) were ≥12 years of age; 51% of patients were female, 55% White, 29% Black or African American, 10% Asian; 53% had an ECOG performance status of 0 and 45% had an ECOG performance status of 1. All patients had prior surgery for ASPS and 55% received at least one prior line of treatment for ASPS; 55% received radiotherapy and 53% received chemotherapy. Of the patients who reported staging at initial diagnosis, all were Stage IV.
Efficacy results of this study are summarized in Table 30.
Table 30: Efficacy Results from Study ML39345 Endpoint All Patients
(N=49)CI: confidence interval; N: number of patients; +: Censored - *
- 95% CI based on Clopper–Pearson exact method.
Overall response rate (95% CI)* 24% (13, 39) Complete Responses, n 0 Partial Responses, n (%) 12 (24) Duration of response Median, month NE (95% CI) (17.0, NE) Range 1+, 41+ Durability of Response ≥6 months, n (%) 8 (67%) ≥12 months, n (%) 5 (42%) - 16 HOW SUPPLIED/STORAGE AND HANDLING
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Immune-Mediated Adverse Reactions
Inform patients of the risk of immune-mediated adverse reactions that may require corticosteroid treatment and interruption or discontinuation of TECENTRIQ, including:
- Pneumonitis: Advise patients to contact their healthcare provider immediately for any new or worsening cough, chest pain, or shortness of breath [see Warnings and Precautions (5.1)].
- Colitis: Advise patients to contact their healthcare provider immediately for diarrhea, blood or mucus in stools, or severe abdominal pain [see Warnings and Precautions (5.1)].
- Hepatitis: Advise patients to contact their healthcare provider immediately for jaundice, severe nausea or vomiting, pain on the right side of abdomen, lethargy, or easy bruising or bleeding [see Warnings and Precautions (5.1)].
- Endocrinopathies: Advise patients to contact their healthcare provider immediately for signs or symptoms of hypophysitis, hyperthyroidism, hypothyroidism, adrenal insufficiency, or type 1 diabetes mellitus, including diabetic ketoacidosis [see Warnings and Precautions (5.1)].
- Nephritis: Advise patients to contact their healthcare provider immediately for pelvic pain, frequent urination, or unusual swelling. [see Warnings and Precautions (5.1)].
- Dermatologic Adverse Reactions: Advise patients to contact their healthcare provider immediately for generalized rash, skin eruption, or painful skin and mucous membrane lesions [see Warnings and Precautions (5.1)].
- Other Immune-Mediated Adverse Reactions: Advise patients to contact their healthcare provider immediately for signs or symptoms of other potential immune-mediated adverse reactions [see Warnings and Precautions (5.1)].
Infusion-Related Reactions
Advise patients to contact their healthcare provider immediately for signs or symptoms of infusion-related reactions [see Warnings and Precautions (5.2)].
Complications of Allogeneic HSCT after PD-1/PD-L1 inhibitors
Follow patients closely for evidence of transplant-related complications and intervene promptly. Consider the benefits versus risks of treatment with a PD-1/PD-L1 blocking antibody prior to or after an allogeneic HSCT [see Warnings and Precautions (5.3)].
Embryo-Fetal Toxicity
Advise females of reproductive potential that TECENTRIQ can cause harm to a fetus and to inform their healthcare provider of a known or suspected pregnancy [see Warnings and Precautions (5.4), Use in Specific Populations (8.1, 8.3)].
Advise females of reproductive potential to use effective contraception during treatment and for at least 5 months after the last dose of TECENTRIQ [see Use in Specific Populations (8.3)].
Lactation
Advise female patients not to breastfeed while taking TECENTRIQ and for at least 5 months after the last dose [see Use in Specific Populations (8.2)].
- SPL UNCLASSIFIED SECTION
-
MEDICATION GUIDE
-
SPL UNCLASSIFIED SECTION
Representative sample of labeling (see the HOW SUPPLIED section for complete listing):
- PRINCIPAL DISPLAY PANEL - 20 mL Vial Carton
- PRINCIPAL DISPLAY PANEL - 14 mL Vial Carton
-
INGREDIENTS AND APPEARANCE
TECENTRIQ
atezolizumab injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:50242-917 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ATEZOLIZUMAB (UNII: 52CMI0WC3Y) (ATEZOLIZUMAB - UNII:52CMI0WC3Y) ATEZOLIZUMAB 1200 mg in 20 mL Inactive Ingredients Ingredient Name Strength HISTIDINE (UNII: 4QD397987E) 62 mg in 20 mL ACETIC ACID (UNII: Q40Q9N063P) 16.5 mg in 20 mL SUCROSE (UNII: C151H8M554) 821.6 mg in 20 mL POLYSORBATE 20 (UNII: 7T1F30V5YH) 8 mg in 20 mL WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:50242-917-01 1 in 1 CARTON 05/18/2016 1 20 mL in 1 VIAL, SINGLE-USE; Type 0: Not a Combination Product 2 NDC:50242-917-86 1 in 1 CARTON 08/11/2016 2 20 mL in 1 VIAL, SINGLE-USE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA761034 05/18/2016 TECENTRIQ
atezolizumab injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:50242-918 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ATEZOLIZUMAB (UNII: 52CMI0WC3Y) (ATEZOLIZUMAB - UNII:52CMI0WC3Y) ATEZOLIZUMAB 840 mg in 14 mL Inactive Ingredients Ingredient Name Strength HISTIDINE (UNII: 4QD397987E) 43.4 mg in 14 mL ACETIC ACID (UNII: Q40Q9N063P) 11.5 mg in 14 mL SUCROSE (UNII: C151H8M554) 575.1 mg in 14 mL POLYSORBATE 20 (UNII: 7T1F30V5YH) 5.6 mg in 14 mL WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:50242-918-01 1 in 1 CARTON 03/08/2019 1 14 mL in 1 VIAL, SINGLE-USE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA761034 03/08/2019 Labeler - Genentech, Inc. (080129000) Establishment Name Address ID/FEI Business Operations Roche Diagnostics GmbH 315028860 ANALYSIS(50242-917, 50242-918) , MANUFACTURE(50242-917, 50242-918) Establishment Name Address ID/FEI Business Operations F. Hoffmann-La Roche Ltd 485244961 MANUFACTURE(50242-917, 50242-918) , ANALYSIS(50242-917, 50242-918) , LABEL(50242-917, 50242-918) , PACK(50242-917, 50242-918) Establishment Name Address ID/FEI Business Operations Genentech, Inc. 833220176 MANUFACTURE(50242-917, 50242-918) , PACK(50242-917, 50242-918) , LABEL(50242-917, 50242-918) , ANALYSIS(50242-917, 50242-918) Establishment Name Address ID/FEI Business Operations F. Hoffmann-La Roche AG 482242971 API MANUFACTURE(50242-917, 50242-918) , ANALYSIS(50242-917, 50242-918) Establishment Name Address ID/FEI Business Operations Genentech, Inc. 146373191 ANALYSIS(50242-917, 50242-918) Establishment Name Address ID/FEI Business Operations Roche Singapore Technical Operation, Pte. Ltd. (RSTO) 937189173 ANALYSIS(50242-917, 50242-918) Establishment Name Address ID/FEI Business Operations Roche Diagnostics GmbH 323105205 API MANUFACTURE(50242-917, 50242-918) , ANALYSIS(50242-917, 50242-918) Establishment Name Address ID/FEI Business Operations Genentech, Inc. 080129000 ANALYSIS(50242-917, 50242-918) , MANUFACTURE(50242-917, 50242-918) , API MANUFACTURE(50242-917, 50242-918) , PACK(50242-917, 50242-918) , LABEL(50242-917, 50242-918)




