Label: ANORECTAL- lidocaine cream
-
Contains inactivated NDC Code(s)
NDC Code(s): 11822-1234-1 - Packager: Rite Aid
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated June 18, 2018
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
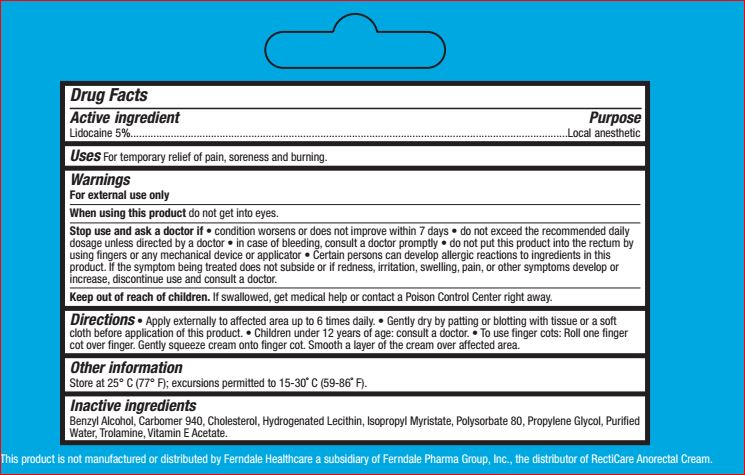
- Active ingredient Purpose
- PURPOSE
- INDICATIONS & USAGE
-
WarningsFor external use only
When using this product do not get into eyes.
Stop use and ask a doctor if • condition worsens or does
not improve within 7 days • do not exceed the
recommended daily dosage unless directed by a doctor
• in case of bleeding, consult a doctor promptly • do not
put this product into the rectum by using fingers or any
mechanical device or applicatorCertain persons can develop allergic reactions to ingredients in this
product. If the symptom being treated does not subside or if redness, irritation, swelling, pain, or other symptoms develop or
increase, discontinue use and consult a doctor -
DOSAGE & ADMINISTRATION
Directions • Apply externally to affected area up to 6 times daily. • Gently dry by patting or blotting with tissue or a soft
cloth before application of this product. • Children under 12 years of age: consult a doctor. • To use finger cots: Roll one finger
cot over finger. Gently squeeze cream onto finger cot. Smooth a layer of the cream over affected area. - OTHER SAFETY INFORMATION
- INACTIVE INGREDIENT
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ANORECTAL
lidocaine creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:11822-1234 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Lidocaine (UNII: 98PI200987) (LIDOCAINE - UNII:98PI200987) Lidocaine 5 g in 100 mL Inactive Ingredients Ingredient Name Strength Benzyl alcohol (UNII: LKG8494WBH) Cholesterol (UNII: 97C5T2UQ7J) HYDROGENATED SOYBEAN LECITHIN (UNII: H1109Z9J4N) Isopropyl Myristate (UNII: 0RE8K4LNJS) Polysorbate 80 (UNII: 6OZP39ZG8H) Propylene Glycol (UNII: 6DC9Q167V3) Water (UNII: 059QF0KO0R) Trolamine (UNII: 9O3K93S3TK) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:11822-1234-1 1 in 1 CARTON 03/24/2016 1 28.3 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part346 03/24/2016 Labeler - Rite Aid (014578892) Registrant - Product Quest (927768135) Establishment Name Address ID/FEI Business Operations Product Quest 927768135 manufacture(11822-1234) , label(11822-1234)