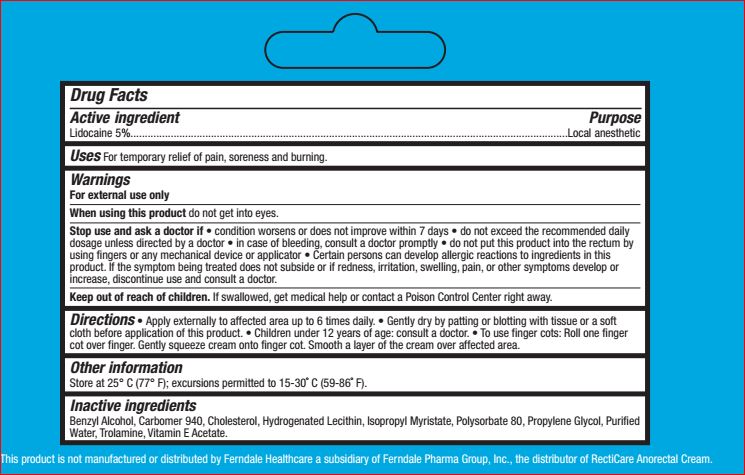
Warnings
For external use only
When using this product do not get into eyes.
Stop use and ask a doctor if • condition worsens or does
not improve within 7 days • do not exceed the
recommended daily dosage unless directed by a doctor
• in case of bleeding, consult a doctor promptly • do not
put this product into the rectum by using fingers or any
mechanical device or applicator
Certain persons can develop allergic reactions to ingredients in this
product. If the symptom being treated does not subside or if redness, irritation, swelling, pain, or other symptoms develop or
increase, discontinue use and consult a doctor
Directions • Apply externally to affected area up to 6 times daily. • Gently dry by patting or blotting with tissue or a soft
cloth before application of this product. • Children under 12 years of age: consult a doctor. • To use finger cots: Roll one finger
cot over finger. Gently squeeze cream onto finger cot. Smooth a layer of the cream over affected area.