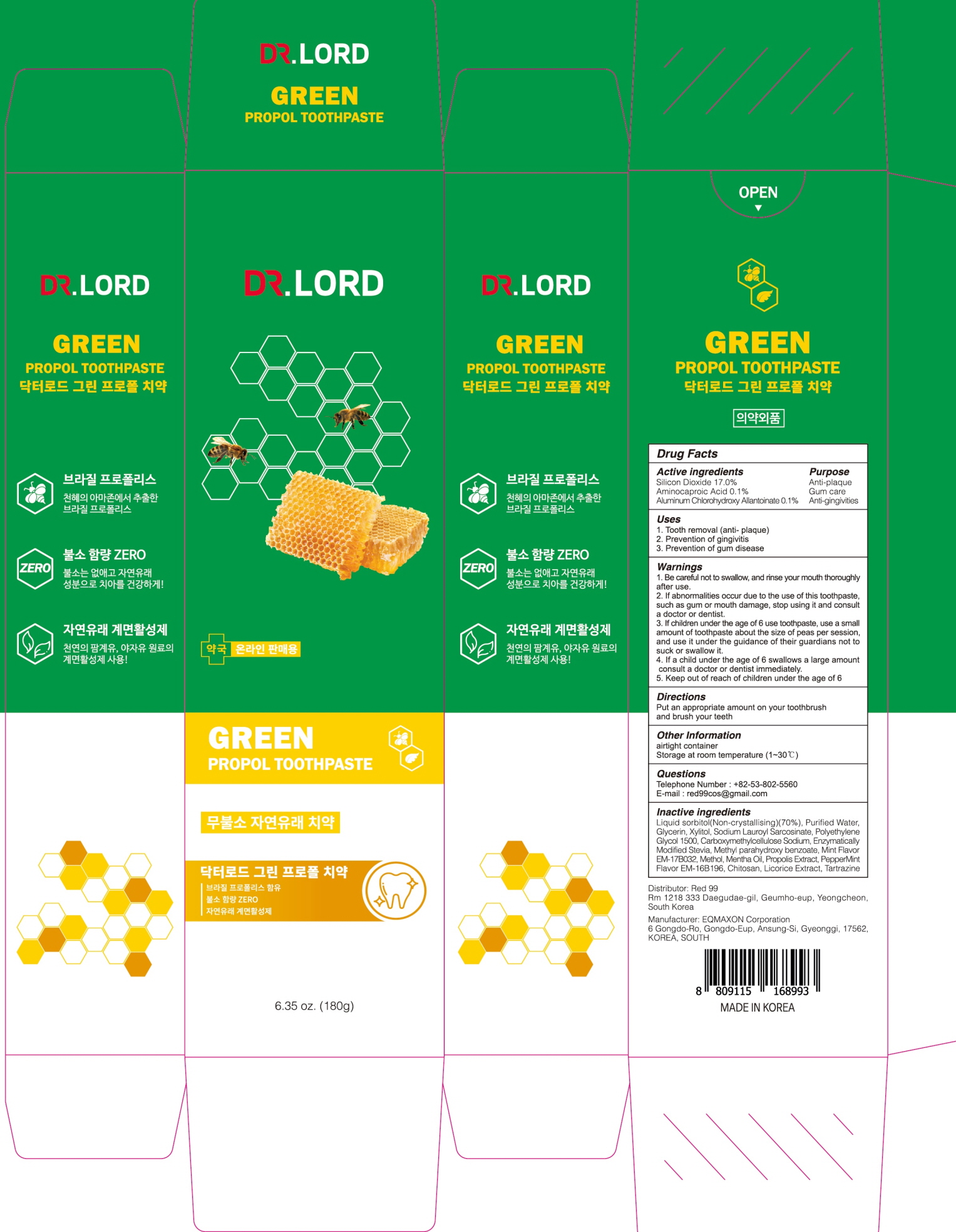
Label: DR.LORD GREEN PROPOL TOOTH- silicon dioxide, aminocaproic acid, aluminum chlorohydroxy allantoinate paste, dentifrice
- NDC Code(s): 82656-010-01, 82656-010-02
- Packager: Red 99
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated April 6, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENTS
-
INACTIVE INGREDIENTS
Liquid sorbitol(Non-crystallising)(70%), Purified Water, Glycerin, Xylitol, Sodium Lauroyl Sarcosinate, Polyethylene Glycol 1500, Carboxymethylcellulose Sodium, Enzymatically Modified Stevia, Methyl parahydroxy benzoate, Mint Flavor EM-17B032, Methol, Mentha Oil, Propolis Extract, PepperMint Flavor EM-16B196, Chitosan, Licorice Extract, Tartrazine
- PURPOSE
-
WARNINGS
1. Be careful not to swallow, and rinse your mouth thoroughly after use.
2. If abnormalities occur due to the use of this toothpaste, such as gum or mouth damage, stop using it and consult a doctor or dentist.
3. If children under the age of 6 use toothpaste, use a small amount of toothpaste about the size of peas per session, and use it under the guidance of their guardians not to suck or swallow it.
4. If a child under the age of 6 swallows a large amount, consult a doctor or dentist immediately.
5. Keep out of reach of children under the age of 6 - KEEP OUT OF REACH OF CHILDREN
- Uses
- Directions
- Other Information
- Questions
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
DR.LORD GREEN PROPOL TOOTH
silicon dioxide, aminocaproic acid, aluminum chlorohydroxy allantoinate paste, dentifriceProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82656-010 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Silicon Dioxide (UNII: ETJ7Z6XBU4) (SILICON DIOXIDE - UNII:ETJ7Z6XBU4) Silicon Dioxide 17.0 g in 100 g Aminocaproic Acid (UNII: U6F3787206) (AMINOCAPROIC ACID - UNII:U6F3787206) Aminocaproic Acid 0.1 g in 100 g ALCLOXA (UNII: 18B8O9DQA2) (ALLANTOIN - UNII:344S277G0Z) ALCLOXA 0.1 g in 100 g Inactive Ingredients Ingredient Name Strength Sorbitol (UNII: 506T60A25R) Water (UNII: 059QF0KO0R) Glycerin (UNII: PDC6A3C0OX) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82656-010-02 1 in 1 CARTON 03/01/2022 1 NDC:82656-010-01 180 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 03/01/2022 Labeler - Red 99 (694767263) Registrant - Red 99 (694767263) Establishment Name Address ID/FEI Business Operations EQMAXON Corp. 557821534 manufacture(82656-010)