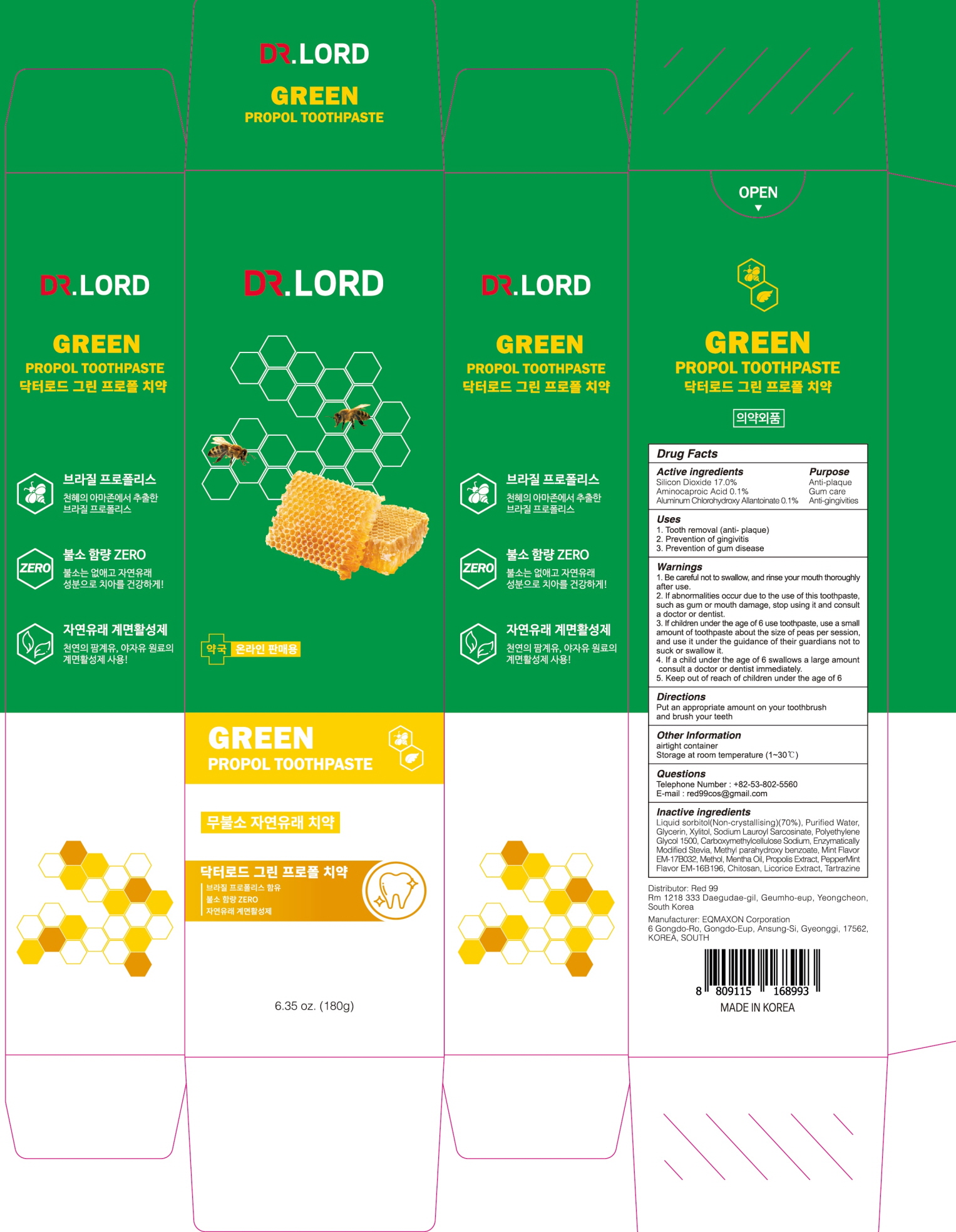
ACTIVE INGREDIENTS
Silicon Dioxide 17.0%
Aminocaproic Acid 0.1%
Aluminum Chlorohydroxy Allantoinate 0.1%
INACTIVE INGREDIENTS
Liquid sorbitol(Non-crystallising)(70%), Purified Water, Glycerin, Xylitol, Sodium Lauroyl Sarcosinate, Polyethylene Glycol 1500, Carboxymethylcellulose Sodium, Enzymatically Modified Stevia, Methyl parahydroxy benzoate, Mint Flavor EM-17B032, Methol, Mentha Oil, Propolis Extract, PepperMint Flavor EM-16B196, Chitosan, Licorice Extract, Tartrazine
WARNINGS
1. Be careful not to swallow, and rinse your mouth thoroughly after use.
2. If abnormalities occur due to the use of this toothpaste, such as gum or mouth damage, stop using it and consult a doctor or dentist.
3. If children under the age of 6 use toothpaste, use a small amount of toothpaste about the size of peas per session, and use it under the guidance of their guardians not to suck or swallow it.
4. If a child under the age of 6 swallows a large amount, consult a doctor or dentist immediately.
5. Keep out of reach of children under the age of 6