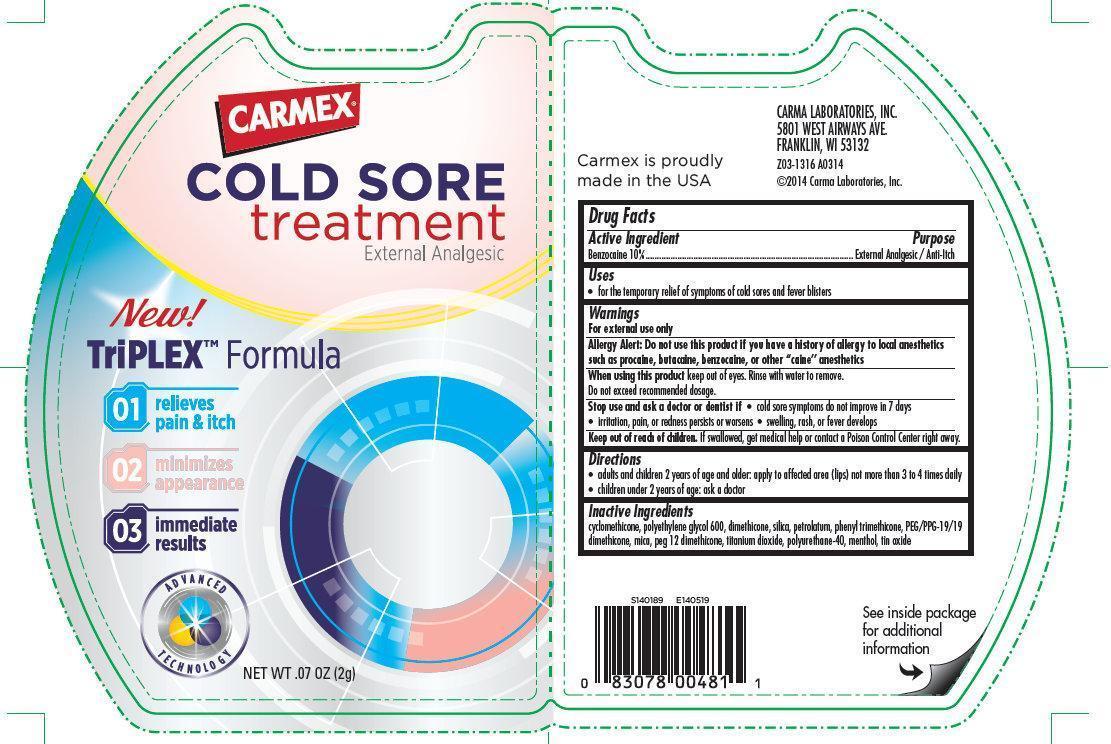
Label: CARMEX COLD SORE TREATMENT EXTERNAL ANALGESIC- benzocaine cream
-
Contains inactivated NDC Code(s)
NDC Code(s): 10210-0012-0 - Packager: Carma Laboratories, Inc.
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated October 21, 2019
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- CARMEX COLD SORE treatment External Analgesic
- Active Ingredient
- Purpose
- Uses
-
Warnings
- For external use only
- Allergy Alert: Do not use this product if you have a history of allergy to local anesthetics such as procaine, butacaine, benzocaine, or other “caine” anesthetics
- When using this product keep out of eyes. Rinse with water to remove.
- Do not exceed recommended dosage.
- Stop use and ask a doctor or dentist if
-
- cold sore symptoms do not improve in 7 days
-
- irritation, pain, or redness persists or worsens
- swelling, rash, or fever develops
- Directions
- Inactive Ingredients
- CARMEX COLD SORE treatment External Analgesic 2g (10210-0012-0)
-
INGREDIENTS AND APPEARANCE
CARMEX COLD SORE TREATMENT EXTERNAL ANALGESIC
benzocaine creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:10210-0012 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZOCAINE (UNII: U3RSY48JW5) (BENZOCAINE - UNII:U3RSY48JW5) BENZOCAINE 10 g in 100 g Inactive Ingredients Ingredient Name Strength CYCLOMETHICONE (UNII: NMQ347994Z) POLYETHYLENE GLYCOL 600 (UNII: NL4J9F21N9) DIMETHICONE (UNII: 92RU3N3Y1O) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) PETROLATUM (UNII: 4T6H12BN9U) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) PEG/PPG-19/19 DIMETHICONE (UNII: EHH90CO7TL) MICA (UNII: V8A1AW0880) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) MENTHOL (UNII: L7T10EIP3A) STANNIC OXIDE (UNII: KM7N50LOS6) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:10210-0012-0 2 g in 1 TUBE; Type 0: Not a Combination Product 05/28/2014 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 05/28/2014 Labeler - Carma Laboratories, Inc. (006090153) Establishment Name Address ID/FEI Business Operations Carma Laboratories, Inc. 006090153 manufacture(10210-0012)