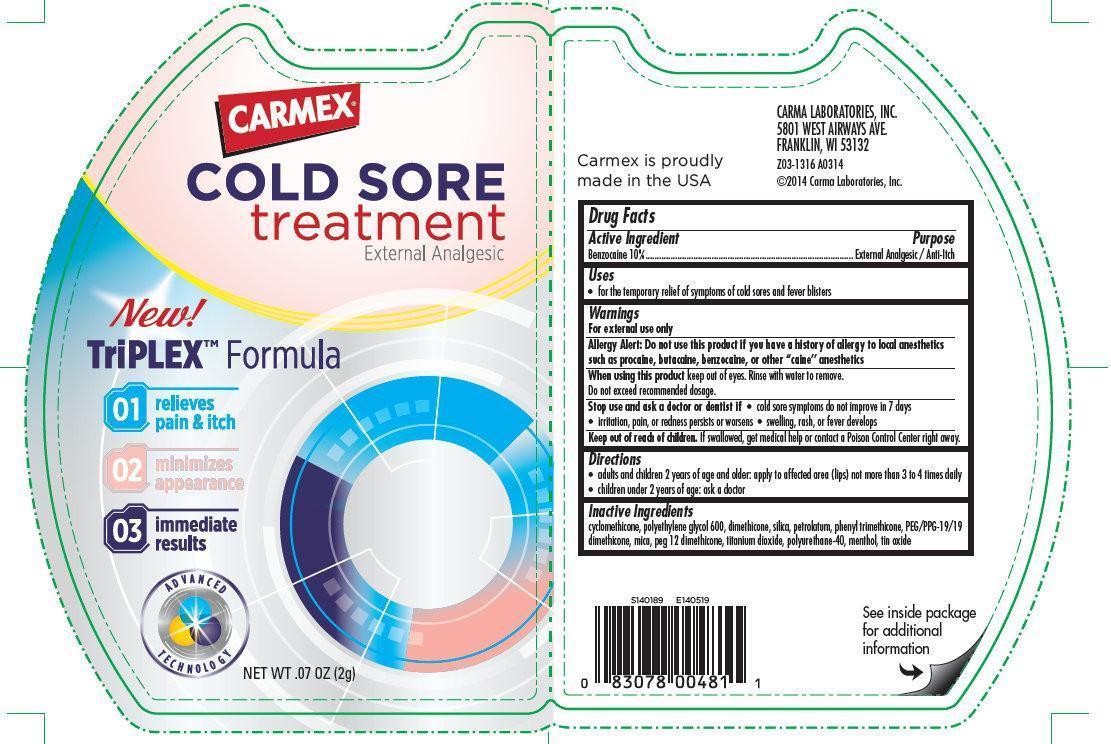
CARMEX COLD SORE TREATMENT EXTERNAL ANALGESIC- benzocaine cream
Carma Laboratories, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
CARMEX COLD SORE treatment External Analgesic
Active Ingredient
Benzocaine 10%
Purpose
External Analgesic / Anti-Itch
Uses
- For the temporary relief of symptoms of cold sores and fever blisters
Warnings
-
For external use only
-
Allergy Alert: Do not use this product if you have a history of allergy to local anesthetics such as procaine, butacaine, benzocaine, or other “caine” anesthetics
-
When using this product keep out of eyes. Rinse with water to remove.
- Do not exceed recommended dosage.
-
Stop use and ask a doctor or dentist if
-
-
cold sore symptoms do not improve in 7 days
-
- irritation, pain, or redness persists or worsens
- swelling, rash, or fever develops
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- adults and children 2 years of age and older: apply to affected area (lips) not more than 3 to 4 times daily
- children under 2 years of age: ask a doctor
Inactive Ingredients
cyclomethicone, polyethylene glycol 600, dimethicone, silica, petrolatum, phenyl trimethicone, PEG/PPG-19/19 dimethicone, mica, peg 12 dimethicone, titanium dioxide, polyurethane-40, menthol, tin oxide
CARMEX COLD SORE treatment External Analgesic 2g (10210-0012-0)
Carma Laboratories, Inc.