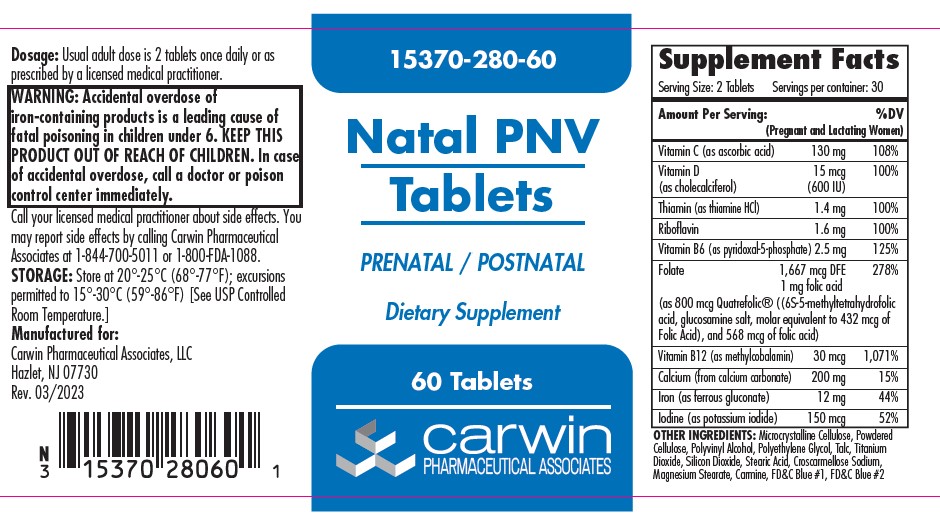
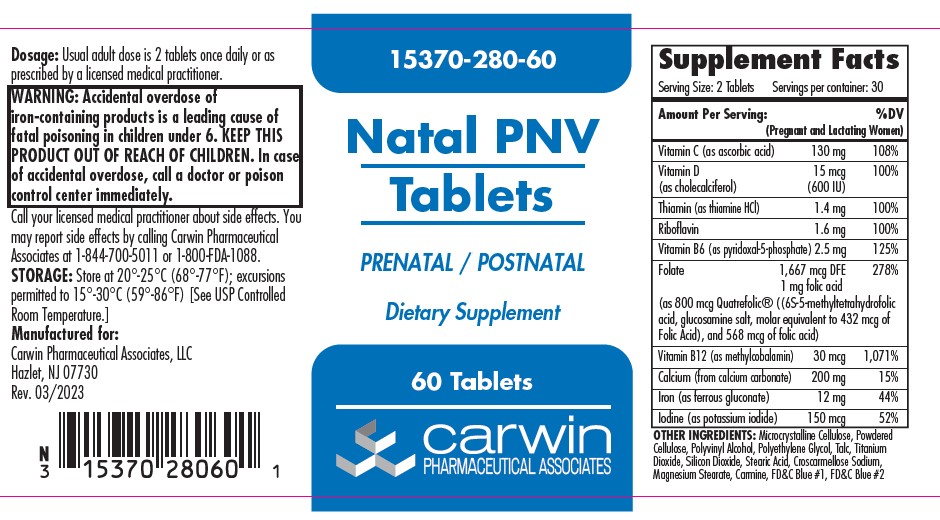
Label: NATAL PNV- ascorbic acid, cholecalciferol, thiamine hydrochloride, riboflavin,pyridoxal phosphate,levomefolate glucosamine, folic acid, methylcobalamin, calcium carbonate, ferrous gluconate, potassium iodide tablet, film coated
- NHRIC Code(s): 15370-280-60
- Packager: Carwin Pharmaceutical Associatates, LLC
- Category: DIETARY SUPPLEMENT
- DEA Schedule: None
- Marketing Status: Dietary Supplement
Drug Label Information
Updated May 4, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- HEALTH CLAIM
-
STATEMENT OF IDENTITY
Serving Size: 2 tablets Servings per container: 30 Amount Per Serving (Pregnant and Lactating Women) %DV Vitamin C (as ascorbic acid) 130 mg 108% Vitamin D (as cholecalciferol) 15 mg (600 U) 100% Thiamin (as thiamin HCl) 1.4 mg 100% Riboflavin 1.6 mg 100% Vitamin B6 (as pyridoxol-5-phosphate) 2.5 mg 125% Folate 1667 mcg, 1 mg folic acid 278% (as 800 mcg Quatrefolic® ((6S-5-methyltetrafolic acid, glucosamine salt, molar equivalent to 432 mcg Folic Acid), and 568 mcg of folic acid) Vitamin B12 (as methylcobalomin) 30 mcg 1,071% Calcium (from calcium carbonate) 200 mg 15% Iron (as ferrous gluconate) 12 mg 44% Iodine (as potassium iodide) 150 mcg 52% OTHER INGREDIENTS: Microcrystalline Cellulose, Powdered Cellulose, Polyvinyl Alcohol, Polyethylene Glycol, Talc, Titanium Dioxide, Silicon Dioxide, Stearic Acid, Croscarmelose Sodium, Magnesium Stearate, Carmine, FD&C Blue #1, FD&C Blue #2
- DESCRIPTION
- CONTRADINDICATIONS
- WARNING
- ADVERSE REACTIONS
- DOSAGE AND ADMINISTRATION
-
PRECAUTION:
Folic acid alone is improper therapy in the treatment of pernicious anemia and other megaloblastic anemias where vitamin B12 is deficient. Folic acid in doses above 0.1 mg daily may obscure pernicious anemia in that hematologic remission can occur while neurological manifestations progress. Natal PNV Tablets should only be used under the direction and supervision of a licensed medical practitioner.
- SAFE HANDLING WARNING SECTION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
NATAL PNV
ascorbic acid, cholecalciferol, thiamine hydrochloride, riboflavin,pyridoxal phosphate,levomefolate glucosamine, folic acid, methylcobalamin, calcium carbonate, ferrous gluconate, potassium iodide tablet, film coatedProduct Information Product Type DIETARY SUPPLEMENT Item Code (Source) NHRIC:15370-280 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ASCORBIC ACID (UNII: PQ6CK8PD0R) (ASCORBIC ACID - UNII:PQ6CK8PD0R) ASCORBIC ACID 130 mg in 2 CHOLECALCIFEROL (UNII: 1C6V77QF41) (CHOLECALCIFEROL - UNII:1C6V77QF41) CHOLECALCIFEROL 15 ug in 2 THIAMINE HYDROCHLORIDE (UNII: M572600E5P) (THIAMINE ION - UNII:4ABT0J945J) THIAMINE 1.4 mg in 2 RIBOFLAVIN (UNII: TLM2976OFR) (RIBOFLAVIN - UNII:TLM2976OFR) RIBOFLAVIN 1.6 mg in 2 PYRIDOXAL PHOSPHATE ANHYDROUS (UNII: F06SGE49M6) (PYRIDOXAL PHOSPHATE ANHYDROUS - UNII:F06SGE49M6) PYRIDOXAL PHOSPHATE ANHYDROUS 2.5 mg in 2 LEVOMEFOLATE GLUCOSAMINE (UNII: Q65PL71Q1A) (LEVOMEFOLATE GLUCOSAMINE - UNII:Q65PL71Q1A) LEVOMEFOLATE GLUCOSAMINE 800 ug in 2 FOLIC ACID (UNII: 935E97BOY8) (FOLIC ACID - UNII:935E97BOY8) FOLIC ACID 568 ug in 2 METHYLCOBALAMIN (UNII: BR1SN1JS2W) (METHYLCOBALAMIN - UNII:BR1SN1JS2W) METHYLCOBALAMIN 30 ug in 2 CALCIUM CARBONATE (UNII: H0G9379FGK) (CALCIUM CATION - UNII:2M83C4R6ZB) CALCIUM CATION 200 mg in 2 FERROUS GLUCONATE (UNII: U1B11I423Z) (FERROUS CATION - UNII:GW89581OWR) FERROUS CATION 12 mg in 2 POTASSIUM IODIDE (UNII: 1C4QK22F9J) (IODIDE ION - UNII:09G4I6V86Q) IODIDE ION 150 ug in 2 Inactive Ingredients Ingredient Name Strength CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) POWDERED CELLULOSE (UNII: SMD1X3XO9M) POLYVINYL ALCOHOL, UNSPECIFIED (UNII: 532B59J990) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) STEARIC ACID (UNII: 4ELV7Z65AP) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) MAGNESIUM STEARATE (UNII: 70097M6I30) CARMINIC ACID (UNII: CID8Z8N95N) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) FD&C BLUE NO. 2 (UNII: L06K8R7DQK) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NHRIC:15370-280-60 60 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date dietary supplement 05/04/2023 Supplement Facts Serving Size : Serving per Container : Amount Per Serving % Daily Value color shape size (solid drugs) 15 mm scoring 1 imprint Labeler - Carwin Pharmaceutical Associatates, LLC (079217215)