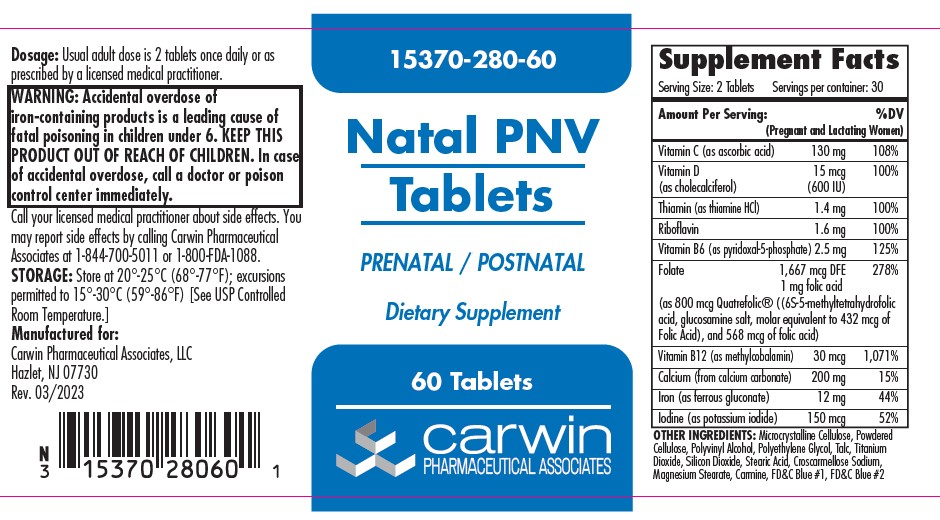
| Amount Per Serving (Pregnant and Lactating Women) %DV |
| Vitamin C (as ascorbic acid) 130 mg 108% |
| Vitamin D (as cholecalciferol) 15 mg (600 U) 100% |
| Thiamin (as thiamin HCl) 1.4 mg 100% |
| Riboflavin 1.6 mg 100% |
| Vitamin B6 (as pyridoxol-5-phosphate) 2.5 mg 125% |
| Folate 1667 mcg, 1 mg folic acid 278% (as 800 mcg Quatrefolic® ((6S-5-methyltetrafolic acid, glucosamine salt, molar equivalent to 432 mcg Folic Acid), and 568 mcg of folic acid) |
| Vitamin B12 (as methylcobalomin) 30 mcg 1,071% |
| Calcium (from calcium carbonate) 200 mg 15% |
| Iron (as ferrous gluconate) 12 mg 44% |
| Iodine (as potassium iodide) 150 mcg 52% |
OTHER INGREDIENTS: Microcrystalline Cellulose, Powdered Cellulose, Polyvinyl Alcohol, Polyethylene Glycol, Talc, Titanium Dioxide, Silicon Dioxide, Stearic Acid, Croscarmelose Sodium, Magnesium Stearate, Carmine, FD&C Blue #1, FD&C Blue #2
DESCRIPTION
Natal PNV Tablets is a prescription dietary supplement for use throughout pregnancy, during the postnatal period for both lactating and non-lactating mothers, and throughout the childbearing years. Natan PNV Tablets may be useful in improving the nutritional status of women prior to conception.
CONTRADINDICATIONS
Natal PNV Tablets are contraindicated in patients with a known hypersensitivity to any of the ingredients. Do not take this product if you are presently taking mineral oil, unless directed by a doctor.
WARNING
Accidental overdose of iron-containing products is a leading cause of fatal poisoning in children under 6. KEEP THIS AND ALL DRUGS OUT OF THE REACH OF CHILDREN. In case of accidental overdose, call a doctor or poison control center immediately.
ADVERSE REACTIONS
Allergic sensitization has been reported following both oral and parenteral administration of folic acid. You may report side effects by calling Carwin Pharmaceutical Associates at 1-844-700-5011 or the FDA by calling 1-800-FDA-1088.
DOSAGE AND ADMINISTRATION
Usual adult dose is 2 tablets taken orally once daily, or as prescribed by a licensed medical practitioner.
PRECAUTION:
Folic acid alone is improper therapy in the treatment of pernicious anemia and other megaloblastic anemias where vitamin B12 is deficient. Folic acid in doses above 0.1 mg daily may obscure pernicious anemia in that hematologic remission can occur while neurological manifestations progress. Natal PNV Tablets should only be used under the direction and supervision of a licensed medical practitioner.