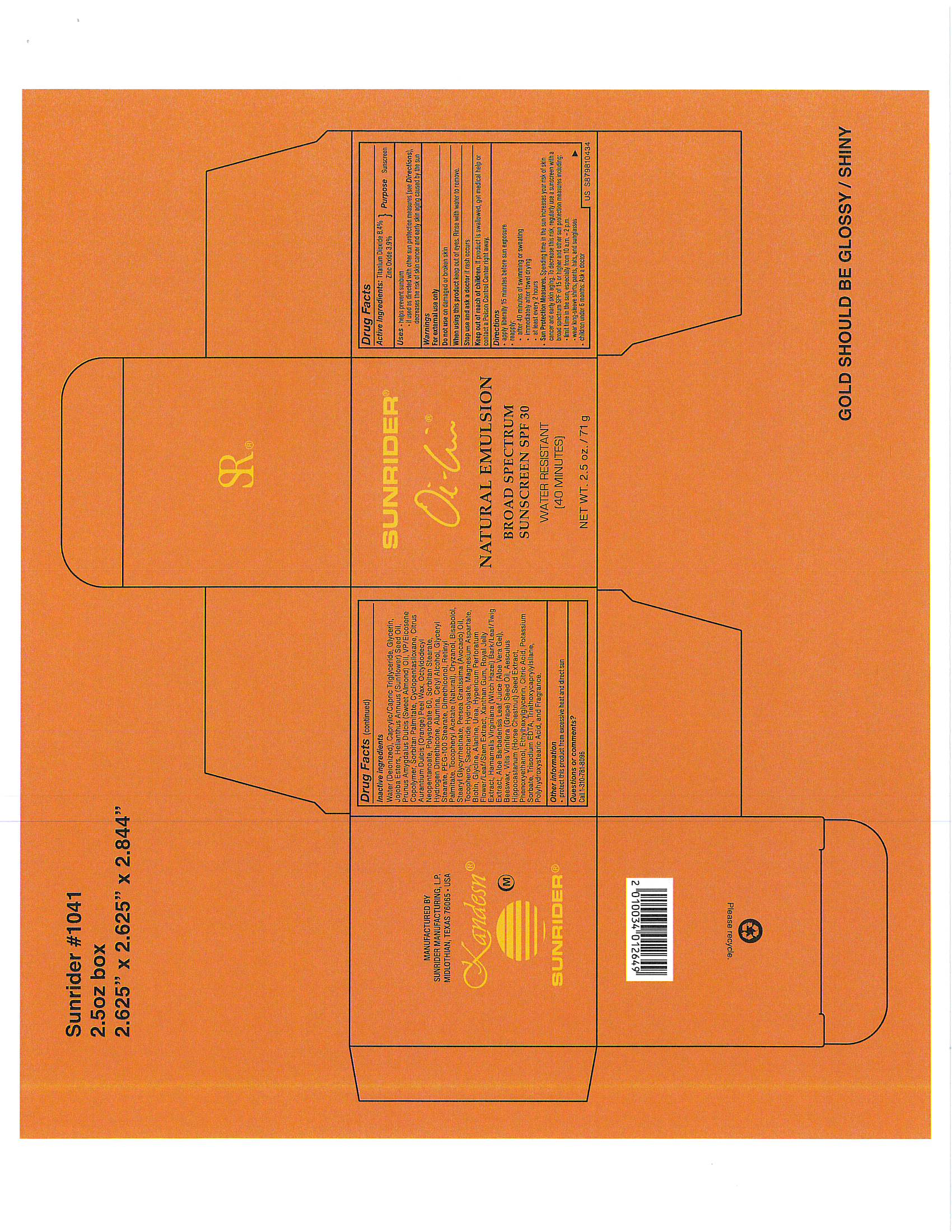
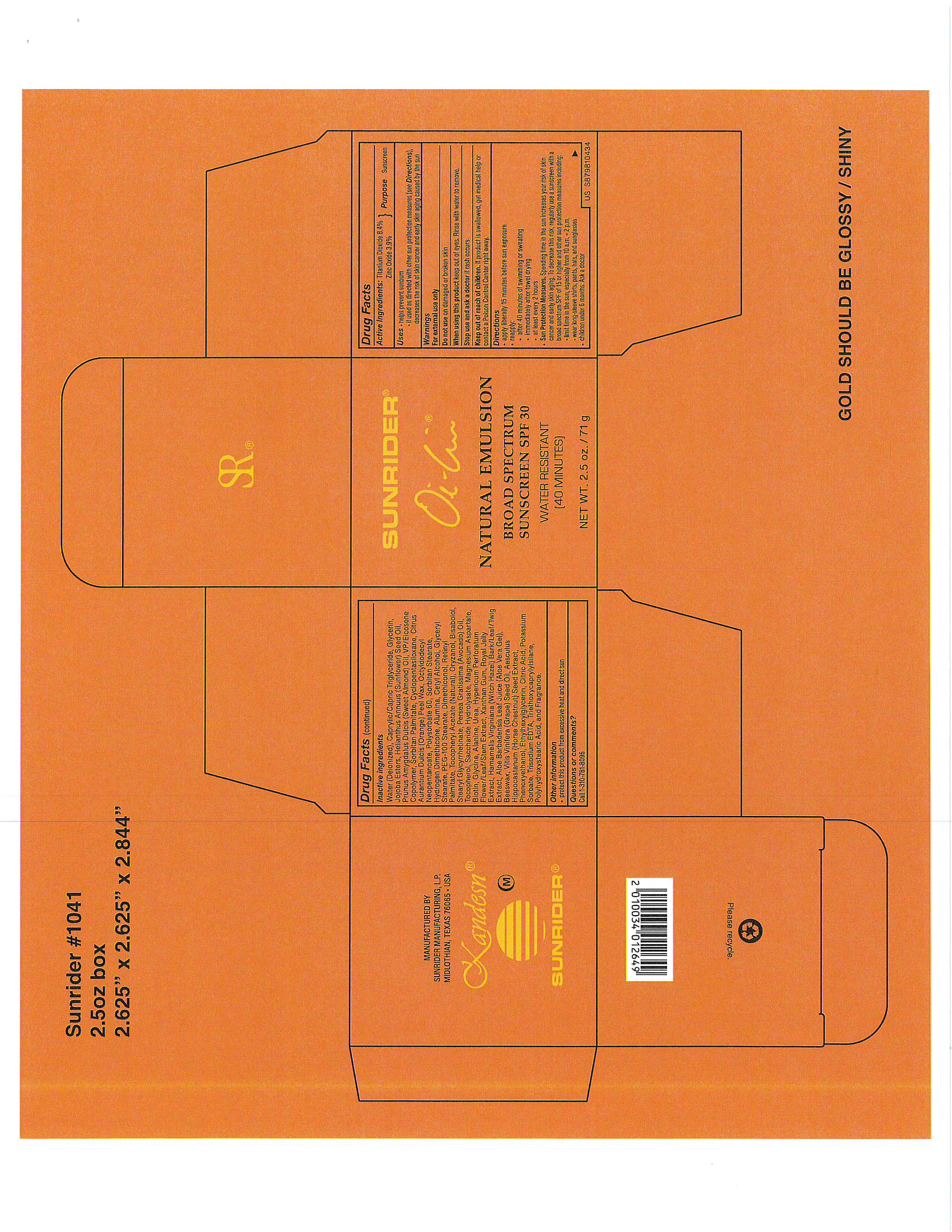
Label: OI LIN NATURAL EMULSION SPF 30- sunscreen, titanium dioxide emulsion
- NDC Code(s): 62191-801-06
- Packager: Sunrider Manufacturing L.P.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 21, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- PURPOSE
-
INDICATIONS & USAGE
Directions:
- Apply liberally 15 minutes before sun exposure.
reapply:
after 40 minutes of swimming or swatingImmediately after towel drying
at least every 2 hours.
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin againg. To decrease this risk, regularly use a sunscreen with a broad spectrum SPF of 15 or higher and other sun protection measures including:
limit time in the sun, expecially from 10 a.m. - 2 p.m.
wear long-sleeve shirts, pants, hats, and sunglasses
Children under 6 months: Ask a doctor - ACTIVE INGREDIENT
-
INACTIVE INGREDIENT
OTHER INGREDIENTS:
Water (Deionized)
Caprylic/Capric Triglyceride
Glycerin
Jojoba Esters
Helianthus Annuus (Sunflower) Seed Oil
Prunus Amygdalus Dulcis (Sweet Almond) Oil
VP/Eicosene Copolymer
Sorbitan Palmitate
Cyclopentasiloxane
Citrus Aurantium Dulcis (Orange) Peel Wax
Polysorbate 60
Octyldodecyl Neopentanoate
Sorbitan Stearate
Cetyl Alcohol
Glyceryl Stearate
PEG-100 Stearate
Dimethiconol
Retinyl Palmitate
Tocopheryl Acetate
Oryzanol
Bisabolol
Stearyl Glycyrrhetinate
Jasminum Officinale Flower Extract
Persea Gratissima (Avocado) Oil
Tocopherol
Zinc Oxide
Saccharide Hydrolysate
Magnesium Aspartate
Biotin
Glycine
Alanine
Urea
Zea Mays (Corn) Oil
Hypericum Perforatum Flower/Leaf/Stem Extract
Xanthan Gum
Royal Jelly Extract
Citrus Medica Limonum Extract
Hamamelis Virginiana (Witch Hazel) Bark/Leaf/Twig Extract
Camellia Sinensis Leaf Extract
Aloe Barbadensis Leaf Juice (Aloe Vera Gel)
Beeswax
Chrysanthemum Indicum Flower Extract
Imperata Cylindrica Root Extract
Pinus Pinaster Bark Extract
Vitis Vinifera (Grape) Seed Oil
Aesculus Hippocastanum (Horse Chestnut) Seed Extract
Phenoxyethanol
Trisodium EDTA
Potassium Sorbate
Ethylhexylglycerin
Citric Acid
Triethoxycaprylylsilane - WARNINGS
- KEEP OUT OF REACH OF CHILDREN
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
OI LIN NATURAL EMULSION SPF 30
sunscreen, titanium dioxide emulsionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:62191-801 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 0.65 g in 1 g Inactive Ingredients Ingredient Name Strength ALANINE (UNII: OF5P57N2ZX) ALMOND OIL (UNII: 66YXD4DKO9) ALOE VERA LEAF (UNII: ZY81Z83H0X) AVOCADO OIL (UNII: 6VNO72PFC1) YELLOW WAX (UNII: 2ZA36H0S2V) Biotin (UNII: 6SO6U10H04) .ALPHA.-BISABOLOL, (+/-)- (UNII: 36HQN158VC) LEVOMENOL (UNII: 24WE03BX2T) Cetyl Alcohol (UNII: 936JST6JCN) CAMELLIA OLEIFERA LEAF (UNII: 5077EL0C60) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) CHRYSANTHEMUM INDICUM TOP (UNII: 607YAE70V1) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) DENDRANTHEMA INDICUM FLOWER (UNII: I6OER6U04L) DIMETHICONE (UNII: 92RU3N3Y1O) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) GAMMA ORYZANOL (UNII: SST9XCL51M) Glycerin (UNII: PDC6A3C0OX) Glycine (UNII: TE7660XO1C) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) GREEN TEA LEAF (UNII: W2ZU1RY8B0) HAMAMELIS VIRGINIANA TOP (UNII: UDA30A2JJY) HORSE CHESTNUT (UNII: 3C18L6RJAZ) HYPERICUM PERFORATUM FLOWER (UNII: A6V4CUE7PV) IMPERATA CYLINDRICA ROOT (UNII: VYT2JA85NH) JASMINUM OFFICINALE FLOWER (UNII: 0Q8K841432) POTASSIUM HYDROLYZED JOJOBA ESTERS (UNII: CH428W5O62) LEMON (UNII: 24RS0A988O) MAGNESIUM ASPARTATE (UNII: R17X820ROL) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) OCTYLDODECYL NEOPENTANOATE (UNII: X8725R883T) ORANGE PEEL WAX (UNII: 0U715N387C) PEG-100 STEARATE (UNII: YD01N1999R) PINUS PINASTER WHOLE (UNII: J27867F361) PHENOXYETHANOL (UNII: HIE492ZZ3T) POLYSORBATE 60 (UNII: CAL22UVI4M) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) ROYAL JELLY (UNII: L497I37F0C) FRUCTOSE (UNII: 6YSS42VSEV) DEXTROSE, UNSPECIFIED FORM (UNII: IY9XDZ35W2) SORBITAN MONOPALMITATE (UNII: 77K6Z421KU) SORBITAN MONOSTEARATE (UNII: NVZ4I0H58X) STEARYL GLYCYRRHETINATE (UNII: 3YYE6VJS0P) SUNFLOWER OIL (UNII: 3W1JG795YI) TOCOPHEROL (UNII: R0ZB2556P8) ALPHA-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) CAPRYLIC/CAPRIC/LAURIC TRIGLYCERIDE (UNII: FJ1H6M2JG9) EDETATE TRISODIUM (UNII: 420IP921MB) UREA (UNII: 8W8T17847W) VITIS VINIFERA SEED (UNII: C34U15ICXA) VINYLPYRROLIDONE/EICOSENE COPOLYMER (UNII: 035MV9S1C3) WATER (UNII: 059QF0KO0R) XANTHAN GUM (UNII: TTV12P4NEE) CORN OIL (UNII: 8470G57WFM) ZINC OXIDE (UNII: SOI2LOH54Z) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:62191-801-06 1 in 1 BOX 10/02/2017 1 71 g in 1 JAR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug part352 10/02/2017 Labeler - Sunrider Manufacturing L.P. (786951475) Registrant - Sunrider Manufacturing L.P. (786951475) Establishment Name Address ID/FEI Business Operations Sunrider Manufacturing L.P. 786951475 manufacture(62191-801)