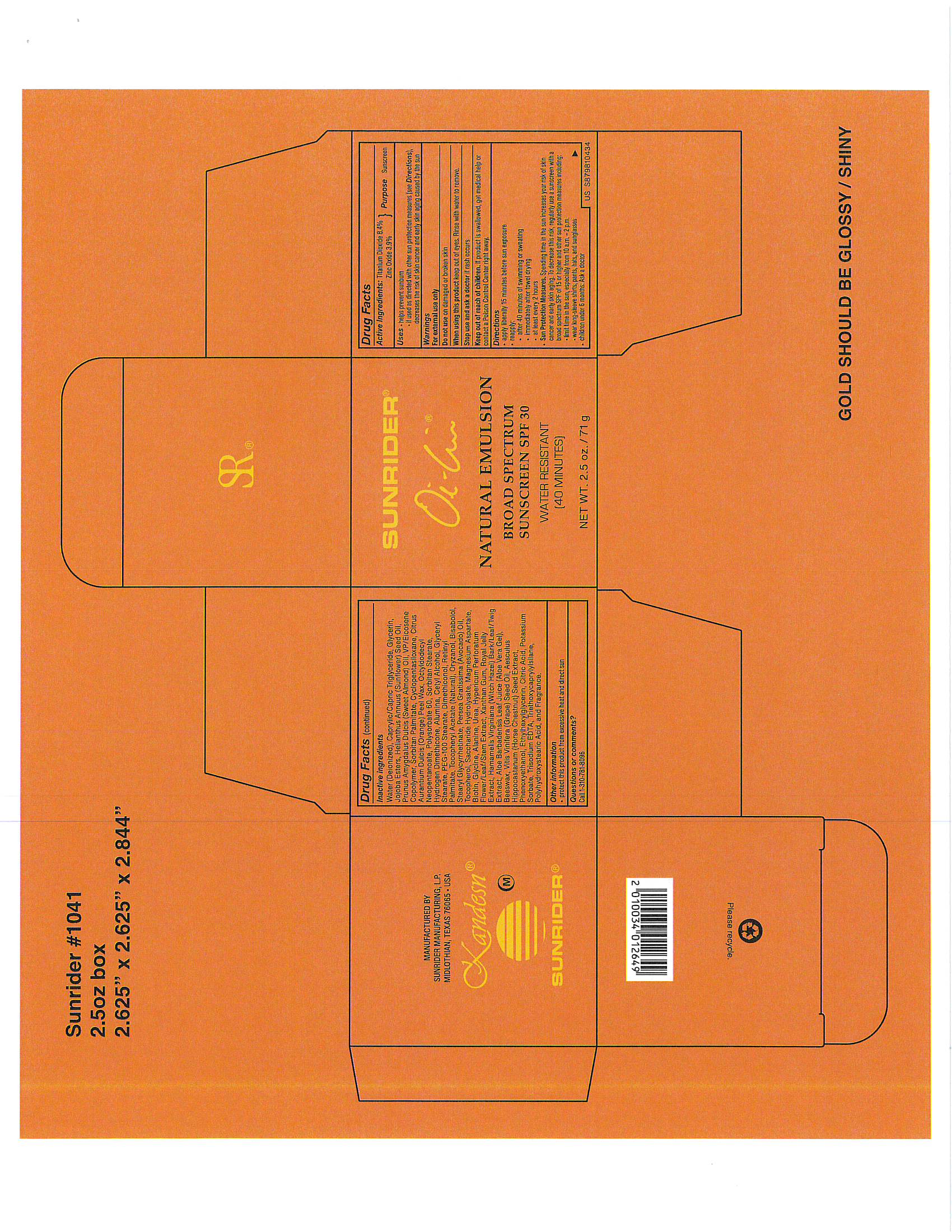
Directions:
- Apply liberally 15 minutes before sun exposure.
reapply:
after 40 minutes of swimming or swating
Immediately after towel drying
at least every 2 hours.
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin againg. To decrease this risk, regularly use a sunscreen with a broad spectrum SPF of 15 or higher and other sun protection measures including:
limit time in the sun, expecially from 10 a.m. - 2 p.m.
wear long-sleeve shirts, pants, hats, and sunglasses
Children under 6 months: Ask a doctor
OTHER INGREDIENTS:
Water (Deionized)
Caprylic/Capric Triglyceride
Glycerin
Jojoba Esters
Helianthus Annuus (Sunflower) Seed Oil
Prunus Amygdalus Dulcis (Sweet Almond) Oil
VP/Eicosene Copolymer
Sorbitan Palmitate
Cyclopentasiloxane
Citrus Aurantium Dulcis (Orange) Peel Wax
Polysorbate 60
Octyldodecyl Neopentanoate
Sorbitan Stearate
Cetyl Alcohol
Glyceryl Stearate
PEG-100 Stearate
Dimethiconol
Retinyl Palmitate
Tocopheryl Acetate
Oryzanol
Bisabolol
Stearyl Glycyrrhetinate
Jasminum Officinale Flower Extract
Persea Gratissima (Avocado) Oil
Tocopherol
Zinc Oxide
Saccharide Hydrolysate
Magnesium Aspartate
Biotin
Glycine
Alanine
Urea
Zea Mays (Corn) Oil
Hypericum Perforatum Flower/Leaf/Stem Extract
Xanthan Gum
Royal Jelly Extract
Citrus Medica Limonum Extract
Hamamelis Virginiana (Witch Hazel) Bark/Leaf/Twig Extract
Camellia Sinensis Leaf Extract
Aloe Barbadensis Leaf Juice (Aloe Vera Gel)
Beeswax
Chrysanthemum Indicum Flower Extract
Imperata Cylindrica Root Extract
Pinus Pinaster Bark Extract
Vitis Vinifera (Grape) Seed Oil
Aesculus Hippocastanum (Horse Chestnut) Seed Extract
Phenoxyethanol
Trisodium EDTA
Potassium Sorbate
Ethylhexylglycerin
Citric Acid
Triethoxycaprylylsilane