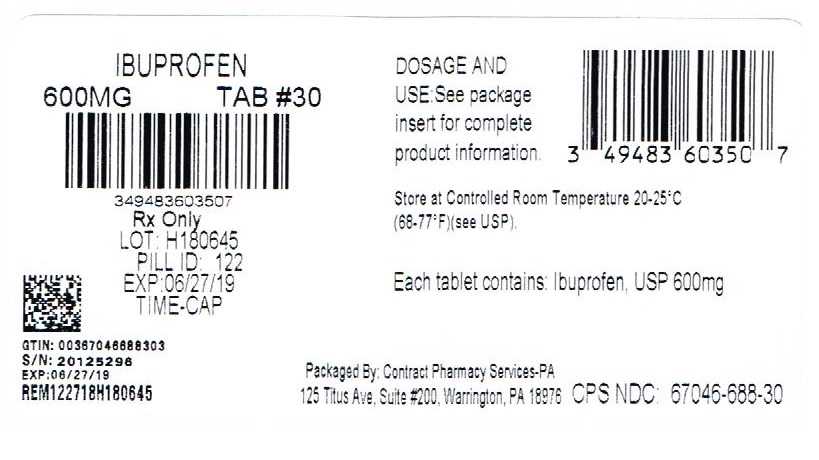
Label: IBUPROFEN tablet, film coated
-
Contains inactivated NDC Code(s)
NDC Code(s): 67046-688-30, 67046-688-60 - Packager: Contract Pharmacy Services-PA
- This is a repackaged label.
- Source NDC Code(s): 49483-603
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated December 29, 2018
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
PATIENT MEDICATION INFORMATION
TIME CAP LABORATORIES, INC TIME CAP LABORATORIES, INC MARKSANS PHARMA LIMITED IBUPROFEN IBUPROFEN IBUPROFEN IBUPROFEN COLLOIDAL SILICON DIOXIDE CROSCARMELLOSE SODIUM MAGNESIUM STEARATE CELLULOSE, MICROCRYSTALLINE POLYETHYLENE GLYCOLS POLYVINYL ALCOHOL STARCH, PREGELATINIZED CORN TALC TITANIUM DIOXIDE 121 IBUPROFEN IBUPROFEN IBUPROFEN IBUPROFEN COLLOIDAL SILICON DIOXIDE CROSCARMELLOSE SODIUM MAGNESIUM STEARATE CELLULOSE, MICROCRYSTALLINE POLYETHYLENE GLYCOLS POLYVINYL ALCOHOL STARCH, PREGELATINIZED CORN TALC TITANIUM DIOXIDE 122 IBUPROFEN IBUPROFEN IBUPROFEN IBUPROFEN COLLOIDAL SILICON DIOXIDE CROSCARMELLOSE SODIUM MAGNESIUM STEARATE CELLULOSE, MICROCRYSTALLINE POLYETHYLENE GLYCOLS POLYVINYL ALCOHOL STARCH, PREGELATINIZED CORN TALC TITANIUM DIOXIDE 123 HOW SUPPLIED 400mg (white to of white, round, biconvex, film coated tablets debossed with '121' on one side and plain on the other side) Bottles of 100 & 500 HOW SUPPLIED 600mg (white to off white, capsule shaped, biconvex, film coated tablets debossed with '122' on one side and plain on the other side) Bottles of 30, 50, 100 & 500 800mg (white to off white, capsule shaped, biconvex, film coated tablets debossed with '123' on one side and plain on the other side) Bottles of 30, 50, 100 & 500 400mg Ibuprofen Package Label image description 600mg Ibuprofen Package Label image description 800mg Ibuprofen Package Label image description REPACKAGED BY: COUPLER ENTERPRISES INC. NDJA 4/19/18
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
IBUPROFEN
ibuprofen tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:67046-688(NDC:49483-603) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength IBUPROFEN (UNII: WK2XYI10QM) (IBUPROFEN - UNII:WK2XYI10QM) IBUPROFEN 600 mg Product Characteristics Color white Score no score Shape CAPSULE Size 18mm Flavor Imprint Code 122 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:67046-688-30 30 in 1 BLISTER PACK; Type 0: Not a Combination Product 02/15/2018 2 NDC:67046-688-60 60 in 1 BLISTER PACK; Type 0: Not a Combination Product 02/15/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA090796 02/15/2018 Labeler - Contract Pharmacy Services-PA (945429777) Establishment Name Address ID/FEI Business Operations Coupler Enterprises Inc. 945429777 repack(67046-688)