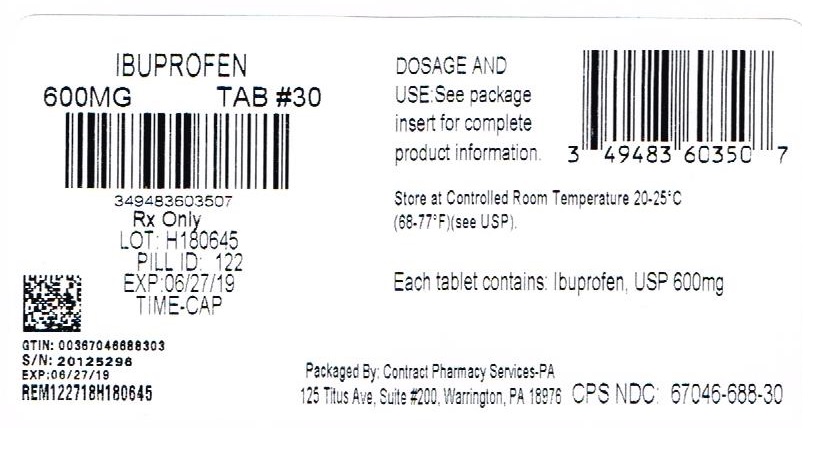
TIME CAP LABORATORIES, INC TIME CAP LABORATORIES, INC MARKSANS PHARMA LIMITED IBUPROFEN IBUPROFEN IBUPROFEN IBUPROFEN COLLOIDAL SILICON DIOXIDE CROSCARMELLOSE SODIUM MAGNESIUM STEARATE CELLULOSE, MICROCRYSTALLINE POLYETHYLENE GLYCOLS POLYVINYL ALCOHOL STARCH, PREGELATINIZED CORN TALC TITANIUM DIOXIDE 121 IBUPROFEN IBUPROFEN IBUPROFEN IBUPROFEN COLLOIDAL SILICON DIOXIDE CROSCARMELLOSE SODIUM MAGNESIUM STEARATE CELLULOSE, MICROCRYSTALLINE POLYETHYLENE GLYCOLS POLYVINYL ALCOHOL STARCH, PREGELATINIZED CORN TALC TITANIUM DIOXIDE 122 IBUPROFEN IBUPROFEN IBUPROFEN IBUPROFEN COLLOIDAL SILICON DIOXIDE CROSCARMELLOSE SODIUM MAGNESIUM STEARATE CELLULOSE, MICROCRYSTALLINE POLYETHYLENE GLYCOLS POLYVINYL ALCOHOL STARCH, PREGELATINIZED CORN TALC TITANIUM DIOXIDE 123 HOW SUPPLIED 400mg (white to of white, round, biconvex, film coated tablets debossed with '121' on one side and plain on the other side) Bottles of 100 & 500 HOW SUPPLIED 600mg (white to off white, capsule shaped, biconvex, film coated tablets debossed with '122' on one side and plain on the other side) Bottles of 30, 50, 100 & 500 800mg (white to off white, capsule shaped, biconvex, film coated tablets debossed with '123' on one side and plain on the other side) Bottles of 30, 50, 100 & 500 400mg Ibuprofen Package Label image description 600mg Ibuprofen Package Label image description 800mg Ibuprofen Package Label image description REPACKAGED BY: COUPLER ENTERPRISES INC. NDJA 4/19/18