Label: ACETAMINOPHEN AND DIPHENHYDRAMINE HYDROCHLORIDE tablet, film coated
-
Contains inactivated NDC Code(s)
NDC Code(s): 65437-041-31, 65437-041-50 - Packager: HIMPRIT PHARMACHEM PVT LTD
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated June 15, 2010
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
- Uses
-
Warnings
Alcohol Warnings
If you consume 3 or more alcoholic drinks every day, ask your doctor if you should take acetaminophen or other pain relievers/fever reducers. Acetaminophen may cause liver damage.
Do not use
- *
- with any other product containing acetaminophen
- *
- with any other product containing diphenhydramine, even one used on skin
- *
- in children under 12 years of age
Ask a doctor before use if you have
- *
- a breathing problem such as emphysema or chronic bronchitis
- *
- glaucoma
- *
- difficulty in urination due to enlargement of the prostate gland
When using this product
- *
- do not exceed recommended dosage
- *
- avoid alcoholic beverages
- *
- marked drowsiness may occur
- *
- do not drive a motor vehicle or operate machinery
Stop use and ask a doctor if
- *
- sleeplessness persists continuously for more than two weeks. Insomnia may be a symptom of a serious underlying medical illness
- *
- new symptoms occur
- *
- redness or swelling is present
- *
- pain gets worse or lasts more than 10 days
- *
- fever gets worse or lasts more than 3 days
-
Direction
- *
- use as directed
- *
- adults and children 12 years and over : take 2 caplets at bedtime or as directed by a doctor
- *
- Children under 12 years : do not use this adult product in children under 12 years of age; this will provide more than the recommended dose (overdose) and could cause serious health problems.
- Other information
- Inactive ingredients
-
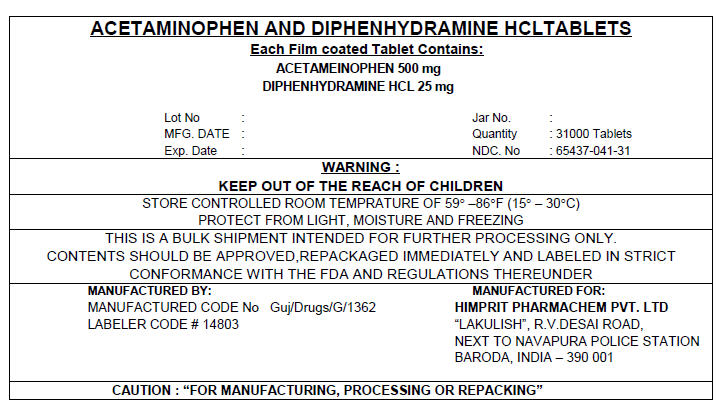
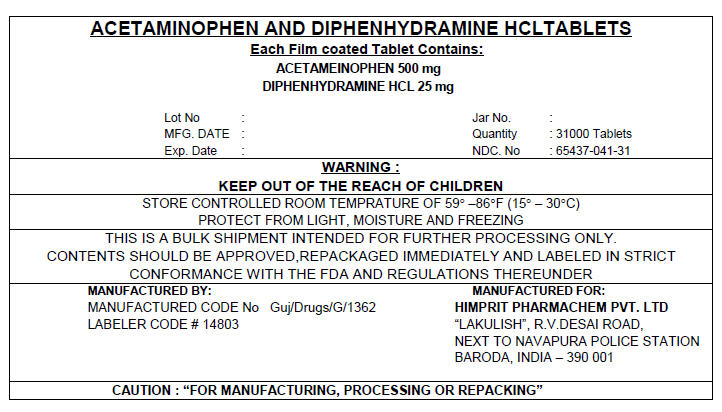
PRINCIPAL DISPLAY PANEL 500/25 mg Shipper Label
ACETAMINOPHEN AND DIPHENHYDRAMINE HCL TABLETS
Each Film coated Tablet Contains:
ACETAMEINOPHEN 500 mg
DIPHENHYDRAMINE HCL 25 mgLot No :
MFG. DATE :
Exp. Date :
Jar No. :
Quantity : 31000 Tablets
NDC. No : 65437-041-31WARNING :
KEEP OUT OF THE REACH OF CHILDRENSTORE CONTROLLED ROOM TEMPRATURE OF 59° –86°F (15° – 30°C)
PROTECT FROM LIGHT, MOISTURE AND FREEZINGTHIS IS A BULK SHIPMENT INTENDED FOR FURTHER PROCESSING ONLY.
CONTENTS SHOULD BE APPROVED,REPACKAGED IMMEDIATELY AND LABELED IN STRICT
CONFORMANCE WITH THE FDA AND REGULATIONS THEREUNDERMANUFACTURED BY:
MANUFACTURED CODE No Guj/Drugs/G/1362
LABELER CODE # 14803MANUFACTURED FOR:
HIMPRIT PHARMACHEM PVT. LTD
"LAKULISH", R.V.DESAI ROAD,
NEXT TO NAVAPURA POLICE STATION
BARODA, INDIA – 390 001CAUTION : "FOR MANUFACTURING, PROCESSING OR REPACKING"
-
INGREDIENTS AND APPEARANCE
ACETAMINOPHEN AND DIPHENHYDRAMINE HYDROCHLORIDE
acetaminophen and diphenhydramine hydrochloride tablet, film coatedProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:65437-041 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 500 mg DIPHENHYDRAMINE HYDROCHLORIDE (UNII: TC2D6JAD40) (DIPHENHYDRAMINE - UNII:8GTS82S83M) DIPHENHYDRAMINE HYDROCHLORIDE 25 mg Inactive Ingredients Ingredient Name Strength SODIUM METABISULFITE (UNII: 4VON5FNS3C) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) STEARIC ACID (UNII: 4ELV7Z65AP) POLYETHYLENE GLYCOL (UNII: 3WJQ0SDW1A) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) WATER (UNII: 059QF0KO0R) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) FD&C BLUE NO. 2 (UNII: L06K8R7DQK) HYPROMELLOSE (UNII: 3NXW29V3WO) TALC (UNII: 7SEV7J4R1U) Product Characteristics Color BLUE Score no score Shape OVAL (Capsule Shaped) Size 18mm Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:65437-041-31 1 in 1 DRUM 1 31000 in 1 BAG 2 NDC:65437-041-50 1 in 1 DRUM 2 50000 in 1 BAG Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part343 07/01/2010 Labeler - HIMPRIT PHARMACHEM PVT LTD (917261992)